Article contents
Electron microscope study of the structure of SrMnO3−x with planar defect
Published online by Cambridge University Press: 08 February 2011
Abstract
Structures of some phases in the SrMnO3−x system accompanied by planar defect were studied using a high resolution electron microscope. A four-layer structure with a hexagonal unit cell was found to have an oxygen deficiency in some degree followed by planar defects normal to the c-axis which are classified into several types. On the basis of observed images showing an irregular stacking along the c-axis in the hexagonal unit, the distribution and the origin of the planar defect are discussed. A structure image showed the transformation of the four-layer phase into a nonstoichiometric state and a shear structure with a displacement vector of 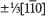 occurring between the face-sharing octahedra. Experiment by in situ observation during beam irradiation in an electron microscope indicates the process of the formation of the planar defect. A mechanism for the formation of the planar defect is proposed by which oxygen deficiency in face-sharing octahedra induces the displacement of SrO3 layers and oxygen octahedra which reduce the coordination number of Mn ions and the disruption of face-sharing octahedra.
occurring between the face-sharing octahedra. Experiment by in situ observation during beam irradiation in an electron microscope indicates the process of the formation of the planar defect. A mechanism for the formation of the planar defect is proposed by which oxygen deficiency in face-sharing octahedra induces the displacement of SrO3 layers and oxygen octahedra which reduce the coordination number of Mn ions and the disruption of face-sharing octahedra.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 1991
References
- 12
- Cited by


