Article contents
Electrodeposition of epitaxial Co(OH)2 on gold and conversion to epitaxial CoOOH and Co3O4
Published online by Cambridge University Press: 23 September 2016
Abstract
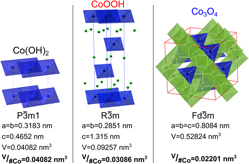
An electrodeposition method for growing epitaxial Co(OH)2 films on single crystalline gold (111), (100), and (110) substrates is described. The films were grown by electrochemical reduction of [Co(en)3]3+ in an alkaline electrolyte. The Co(OH)2 grew with a [0001] out-of-plane orientation on all the gold crystal orientations. The in-plane orientation follows the symmetry of the gold (111), (100), and (110) substrates. The Co(OH)2 can be converted to CoOOH by electrochemical oxidation in 1 M KOH at 95 °C, and after conversion remains epitaxial with a [0001] out-of-plane orientation. The CoOOH film can be further converted to epitaxial Co3O4 with a [111] out-of-plane orientation by decomposition of the CoOOH film in air at 300 °C. This synthesis method allows for a simple fabrication of epitaxial catalysts and could be useful to probe the catalytic activity of specific crystal planes.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
Footnotes
These authors contributed equally to this work.
Contributing Editor: Edson Roberto Leite
References
REFERENCES
- 9
- Cited by



