Article contents
Direct coagulation casting of alumina via controlled release of calcium from ammonium polyphosphate chelate complex
Published online by Cambridge University Press: 13 January 2016
Abstract
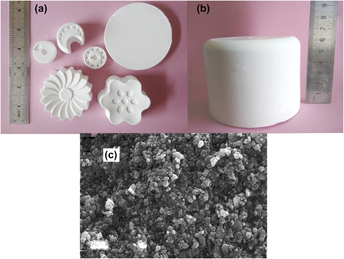
Direct coagulation casting of alumina via controlled release of high valence counter ions using ammonium polyphosphate (APP) chelate complex as the coagulating agent was proposed. APP chelate complex suspension was prepared from APP and calcium chloride. Calcium used as high valence counter ions was chelated by APP. The average particle size of the chelate complex is 0.13 μm with a narrow particle size distribution which is close to the size of alumina particles. Glycerol diacetate was used to tailor the pH value of the suspension by hydrolysis which produces acetic acid. The lowering of the pH value helps to decompose the chelate complex, and enhance to release the calcium chelated. It is indicated that the viscosity of the suspensions with the addition of APP chelate complex suspension and glycerol diacetate increases to approximately 20 Pa s after heating at 40–70 °C for 1.5–5 h, which is high enough to coagulate the suspension. 55 vol% alumina suspension with a addition of 3 vol% APP chelate complex suspension and 5 vol% glycerol diacetate treated at 60 °C could coagulate completely within 2 h with a compressive strength of 2.1 MPa. Dense alumina with a relative density of above 97% and a flexural strength of 388 ± 23 MPa can be prepared by this method from 55 vol% alumina suspensions without a burnout process.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 31 , Issue 1: Annual Issue: Early Career Scholars in Materials Science , 14 January 2016 , pp. 154 - 162
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 4
- Cited by


