Published online by Cambridge University Press: 30 May 2012
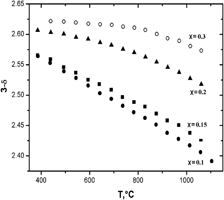
Samples with overall composition HoxSr1−xCoO3−δ within the range 0.05 ≤ x ≤ 0.9 were prepared by a solid-state technique at 1100 °C in air. Single-phase HoxSr1−xCoO3−δ oxides were obtained within the range 0.05 ≤ x ≤ 0.30. Solid solutions of Ho0.05Sr0.95CoO3−δ and Ho0.1Sr0.9CoO3−δ were indexed in the cubic structure (Pm3m sp. gr.) with the unit cell parameters a = 3.846 Å and a = 3.842 Å, respectively. Further introduction of holmium leads to a change of crystal structure from cubic to a tetragonal 2ap × 2ap × 4ap superstructure. All samples with x > 0.3 were multiphase, containing a saturated solid solution with approximate composition Ho0.3Sr0.7CoO3−δ with Ho2O3 and CoO. The change of oxygen nonstoichiometry was measured by thermogravimetric analysis within the temperature range 25 ≤ T (°C) ≤ 1100. The absolute value of oxygen nonstoichiometry was calculated from the results of chromatometric titration. Thermal expansion coefficients of Ho1−xSrxCoO3−δ were measured by dilatometry within the temperature range 25 ≤ T (°C) ≤ 1100 in air.