Published online by Cambridge University Press: 22 September 2016
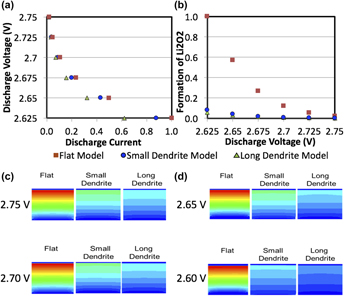
Among the different energy storage technologies under study, lithium–oxygen batteries are one of the most promising due to their great gravimetric energies and capacities 6–10 times greater than other technologies such as conventional lithium-ion cells. The current study provides a comprehensive understanding of how the anodic (e.g., dendrites) and cathodic designs (e.g., porosity of the carbon cathode and mass fraction of oxygen) affect the discharge characteristics of lithium–oxygen cells. When comparing all changes in dendrite surface, porosity and oxygen restriction, it is concluded that although the changes in porosity and oxygen decrease the performance of the cells, the dendrites led to the greatest decrease in performance of the battery when examining the capacity of the cell. This comprehensive understanding will aid in the design of a cyclable and commercially viable lithium–oxygen battery that could be used for a wide range of energy storage applications.