Published online by Cambridge University Press: 19 May 2020
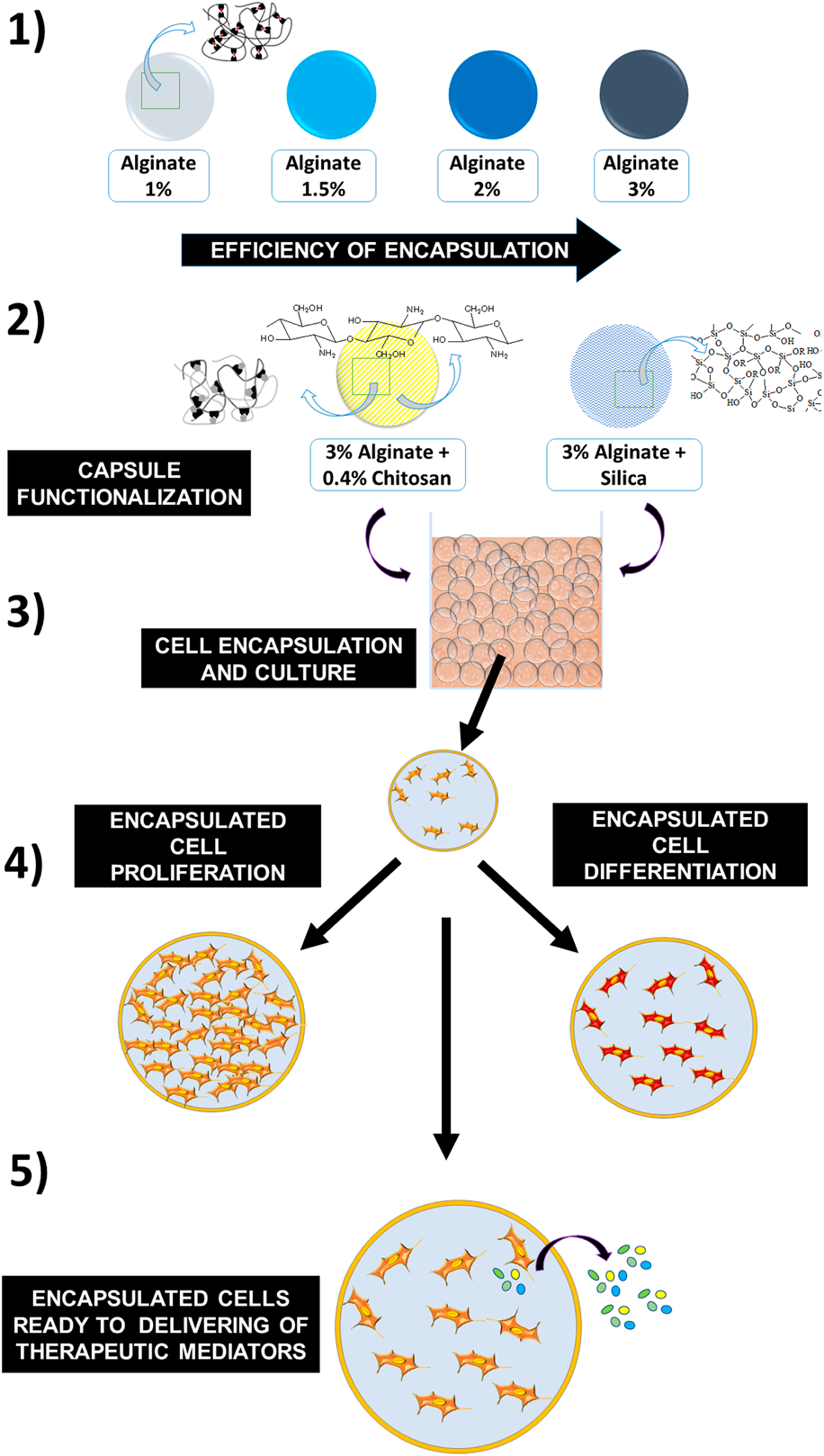
Microencapsulation of functioning cells for transplantation therapies is particularly promising, but the cells must retain their proper physiology and viability after being encapsulated. K-562 cells are multipotential and exhibit erythroid, megakaryocytic, or granulocytic properties that can be exploited by using an array of physiologically differentiating factors. The potential for cell differentiation makes it attractive the use of K-562 cells as functional model to the assessment of the effects of encapsulation on cell viability and physiology. Thus, alginate and hybrid alginate matrices were produced by extrusion technique for K-562 cell encapsulation. The produced systems were composed of bare alginate (1–3 wt%) and alginate in combination with chitosan or silica. The resulting materials were characterized by dynamic laser scattering, zeta potential measurements, small-angle X-ray scattering, and Fourier transform infrared spectroscopy. To assess viability, the encapsulated cells were subjected to the Trypan blue exclusion technique and NAD(P)H-dependent oxidoreductase (MTT) assays; hemin-induced erythroid differentiation capacity was also evaluated. The encapsulated alginate-based systems were shown to be monomodal and bimodal, depending on the nature of the capsule, with mean sizes in the range between 414 and 4.129 nm. Encapsulated cells exhibited viability ratios compatible with their use for prolonged cell cultures. Erythroid differentiation occurred in the range between 39 and 44%. The present results allow the consideration of the viability of therapeutic cells encapsulated in both bare alginate and in hybrid matrices.