Published online by Cambridge University Press: 01 February 2013
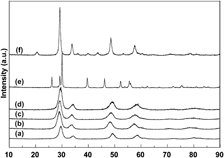
Ce0.35Zr0.65−xRExO2 (RE = Y and La; x = 0 and 0.10) and Ce0.35Zr0.50Y0.075La0.075O2 were prepared by a coprecipitation method. The textures, structures, oxygen storage capacity (OSC), and redox properties of all samples were investigated using Brunauer–Emmett–Teller surface area characterization, x-ray diffraction (XRD), Raman spectra, temperature-programmed technique, and oxygen pulse reaction. The results showed that the fresh Ce0.35Zr0.65O2 has cubic phase, 434 μmol/g of OSC, 82 m2/g of surface area, and good redox properties; after aging at 1000 °C, Ce0.35Zr0.65O2 still has cubic phase, 418 μmol/g of OSC, and 50 m2/g of surface area; when Y3+ or La3+ is added to CeO2–ZrO2, the aged Ce0.35Zr0.65−xRExO2 (RE = Y and La; x = 0 and 0.10) still remains cubic phase, high OSC, and large surface area (47 m2/g); when Y3+ and La3+ are simultaneously added into CeO2–ZrO2, a stable solid solution with cubic phase is formed and has 459 μmol/g of OSC; and the aged Ce0.35Zr0.50Y0.075La0.075O2 reaches to 60 m2/g of surface area and has 390 μmol/g of OSC.