Published online by Cambridge University Press: 16 April 2015
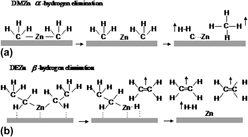
In this study, the authors have comparatively studied the influence of H2 addition on the structures and properties of ZnO films grown by metal organic (MO) chemical vapor deposition with dimethyl zinc and diethyl zinc as zinc precursors and N2O and O2 as oxygen sources, respectively. Various characterization methods, like x-ray diffraction, Raman scattering, Hall effect, photoluminescence, and atomic force microscopy, have been utilized, showing that H2 has different effects on different MO precursors and oxidants. The H2 addition has significantly improved the crystal structural quality of ZnO thin films for the case of dimethyl zinc source, but an opposite effect has been found for the case of diethyl zinc. Moreover, the H2 addition can significantly improve the optical properties of the ZnO films, regardless of the zinc MO sources used, with the surface morphology improved too. The suppression of carbon-related contaminations depends on the use of different precursors and whether H2 is added. By analyzing the experimental results, we have given the effects of H2 on the decomposition of the discussed MO precursors and oxidants, the proposed mechanism could be used in understanding the experimental data.