Published online by Cambridge University Press: 27 June 2011
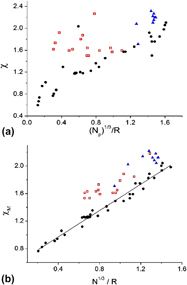
Following our recent report demonstrating the significance of the nearest-neighbor unlike atom-pair bond in metallic alloys, an equation for the energy of such a bond is presented in this study. The success of empirically derived Miedema’s equation in predicting the signs of the heats of formation of metallic alloys is explained. The negative contribution to the energy stems from the ionicity in the bond. The charge transfer on the bond, suggested by Pauling to establish electroneutrality, contributes the positive term which is quantified in this study. The value of Miedema’s empirically derived constant (Q/P)1/2 and the origin of the R/P term follow from the present model. It is shown that the energy of the atom-pair bond, calculated using the model, in the compounds of MgCu2 structure type are directly correlated to the experimental heats of formation of the compounds, and this fact enables the prediction of the heats of formation values for new compounds of the same structure type.