Published online by Cambridge University Press: 16 September 2014
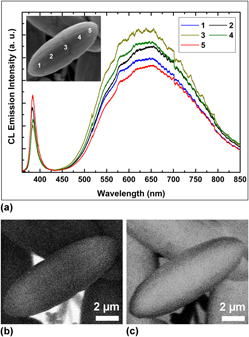
A facile and reproducible low-temperature (80 °C) solution route has been introduced to synthesize ZnO ellipsoids on silicon substrate without any pretreatment of the substrate or organic/inorganic additives. Scanning electron microscopy, transmission electron microscopy, and x-ray diffraction spectroscopy are performed to analyze the structural evolution, the single crystalline nature, and growth orientation at different stages of the synthetic process. The sequential formation mechanisms of heterogeneous nucleation in primary and secondary crystal growth behaviors have been discussed in detail. The presented results reveal that the morphology of micro/nanostructures with desired features can be optimized. The optical properties of grown structures at different stages were investigated using cathodoluminescence (CL). The monochromatic CL images were recorded to examine the UV and visible band emission contributions from the different positions of the intermediate and final structures of the individual ZnO ellipsoid. Significant enhancement in the defect level emission intensity at the central position of the structure reveals that the quality of the material improves as the reaction time is extended.