Article contents
Biomineralization in human pancreas: A combined infrared-spectroscopy, scanning electron microscopy, x-ray Rietveld analysis, and thermogravimetric study
Published online by Cambridge University Press: 21 December 2015
Abstract
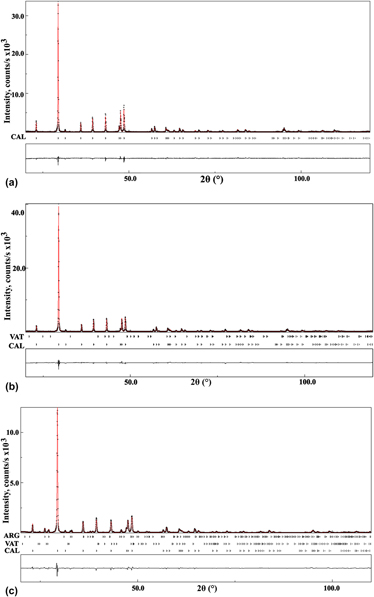
Structural characterization, quantitative phase analysis, and morphological behavior of biomineralized deposits in human pancreas [pancreatic stones (PSs)] have been carried out using infrared (IR)-spectroscopy, scanning electron microscopy (SEM), powder x-ray diffraction, and thermogravimetry - differential scanning calorimetry (TG–DSC). The fourier transform infrared (FT-IR) spectra indicated that the primary composition of PSs was calcium carbonate. An x-ray powder diffraction phase quantification using the Rietveld method revealed that five of the pancreatic calculi were composed exclusively of calcite (CAL) and the remaining four contained small amounts of vaterite and aragonite in addition to the CAL phase. The crystallite size of CAL in the PSs study varied between 104(6) and 181(2) nm. The SEM images of pancreatic calculi showed a variety of crystal morphologies for biogenic CAL crystallites such as, thin plates, spherulites, prisms, and cylindrical laths. Thermogravimetric analysis of PS1 reveals that biogenic CAL is stable up to 910 K, above which temperature CAL transforms into calcium oxide.
Keywords
- Type
- Biomineralization and Biomimetics Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
Footnotes
Contributing Editor: Colin Freeman
References
REFERENCES
- 3
- Cited by


