Published online by Cambridge University Press: 05 December 2019
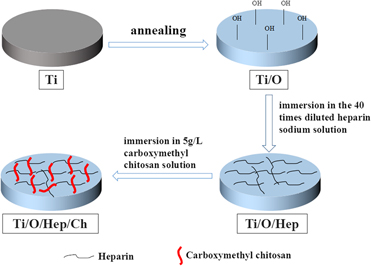
Titanium (Ti) has been extensively used in medical devices owing to their low density, high strength, excellent corrosion resistance, and biocompatibility. However, thrombus formation and bacterial infections are still challenges for their application in specific clinical cases. Hence, we have developed a simple, efficient, and stable strategy that endow Ti with anticoagulant and antibacterial properties through chemical bonding and electrostatic bonding. A large number of hydroxyl groups were produced on the surface of Ti by annealing at 500 °C. Then, heparin was immobilized on annealed surface with chemical bonding and chitosan was captured in an electrostatically bound manner by simply soaking in solution. The results indicated that the surface-functionalized Ti exhibited excellent anticoagulant properties by a reduction in platelet adhesion and prolonged blood clotting time. Furthermore, the modified Ti also showed antibacterial properties against both Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus).