Published online by Cambridge University Press: 05 January 2017
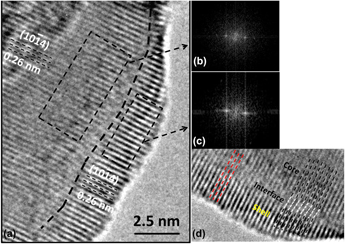
Magnetic core–shell nanoparticles (CSNs) have potential applications in spintronic devices, drug delivery systems, and magnetic random access memory. By use of our hydrothermal nano-phase epitaxy method, we have accomplished synthesis of novel, well-ordered α-Cr2O3@α-Mn0.35Cr1.65O2.94 inverted CSNs. XRD and TEM analyses show a core–shell structure with corundum phase throughout the core and shell with a minimal amount of interface defects. TEM-EDX and XPS data show Mn having the +2 oxidation state in the shell of the CSNs. Magnetization measurements at 5 K show a weak coercivity (H C) value of 8 Oe and an exchange bias field (H E) of 293 Oe. Ab initio calculations show that Mn incorporation in α-Cr2O3 results in narrowing of the energy band gap, substantiated by UV–Vis measurements, and half metallic behavior in case of Mn(III) substitution. Our calculations substantiate that Mn substitution in α-Cr2O3 results in a combination of antiferromagnetic and weak ferrimagnetic character of our CSNs.