Published online by Cambridge University Press: 18 August 2020
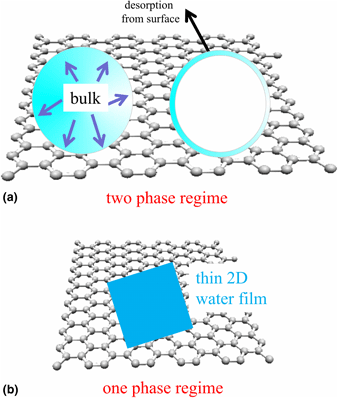
Graphene and its functionalization are still one of the most prominent two-dimensional crystals. In recent years, the wetting properties of graphene for water (i.e., its hydrophobic, hydrophilic, and also icophobic features) were controversially discussed as well as water intercalation and confined water, that have unusual characteristics. The dispute about wetting properties was originally based on contact angle (/engineering) measurements conducted at ambient pressure. In the meanwhile, detailed ultra-high vacuum (UHV) surface science works and theoretical studies are available. This brief review describes the current knowledge available in the literature about the water/graphene system as well as our own work using experimental UHV surface science techniques. The review starts with a definition of hydrophobicity and briefly touches on a possible correlation with icephobicity as well as discusses briefly confined water. Next, theoretical studies are reviewed, and finally, experimental works are described on which the review focusses. Finally, a brief outlook section discusses water adsorption on functionalized graphene.
Please note an has been issued for this article.