Published online by Cambridge University Press: 16 July 2019
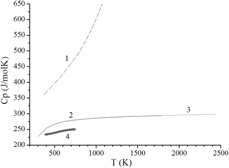
Using the data obtained by Knudsen effusion mass spectrometry, the standard formation thermodynamic properties of La2Hf2O7, Nd2Hf2O7, and Gd2Hf2O7 were calculated in the present study at high temperatures. Based on the results obtained, it was shown that the standard formation Gibbs energies of La2Hf2O7, Nd2Hf2O7, and Gd2Hf2O7 from the elements at the temperature 2445 K were consistent with the empirical rule concerning decrease of stability of pyrochlore hafnate phase with decrease in lanthanoid ionic radius. The La2Hf2O7 and Gd2Hf2O7 heat capacities were obtained in the present study by differential scanning calorimetry. These data were used along with those found earlier to evaluate the standard formation Gibbs energies of La2Hf2O7 and Gd2Hf2O7 from the elements at the temperature 298 K, which equal (−3937 ± 10) kJ/mol and (−3895 ± 10) kJ/mol, respectively. The thermodynamic properties of La2Hf2O7, Nd2Hf2O7, and Gd2Hf2O7 estimated in a wide temperature range allowed consideration of reliability of data available in the literature.