Published online by Cambridge University Press: 03 October 2016
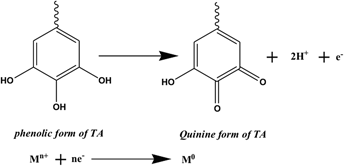
An eco-friendly, green aqueous technique for the preparation of NiO–Ag bimetallic and its individual monometallic nanoparticles (NPs) is succinctly described by utilizing nontoxic and abundantly available tannic acid at room temperature. The so-synthesized nanoscale particles were characterized using various techniques including HRTEM, DLS, zeta potential, SAED, SEM, EDAX, XRD, IR, and UV–vis spectroscopy. These monometallic and bimetallic NPs have a narrow size distribution with spherical morphology. Moreover, the average diameters of all these three different NPs are almost identical and ranges from 7 nm to 10 nm as measured from HRTEM. DLS readings further confirm that the so synthesized particles are in nano range. A comparative catalytic efficacy of the ensuing nanoparticulate materials were assayed employing photodegradation and chemical reduction of methyl violet (MV) at room temperature. NiO–Ag NPs exhibits higher catalytic potential and it took only 15 min to completely reduce MV in presence of NaBH4. The rate constants for both the chemical reduction and photodegradation reactions follow the order: kNiO–Ag bimetallic NPs > kNiO NPs > kAg NPs > kuncat. Higher catalytic performance of the bimetallic system is reckoned on composition effect which basically results due to synergistic electronic effect.
Contributing Editor: Jürgen Eckert