Published online by Cambridge University Press: 08 August 2013
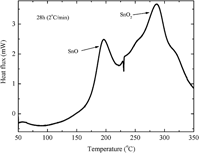
The effects of stearic acid on the high-energy ball milling of tin powder have been investigated. The mean crystallite sizes, microstrain, and phase transformations were examined using different techniques like x-ray diffraction (XRD), Rietveld refinement method, and differential scanning calorimetry (DSC). After 28 h of milling, the Rietveld analysis showed the stabilization of Sn mean crystallite sizes at around 50 nm. Due to the presence of oxygen in stearic acid, the milling process gradually produced an amorphous Sn oxide phase. The DSC thermogram of the sample milled for 28 h showed two exothermic peaks separated by an endothermic peak. Based on the DSC measurements, two samples were annealed at 240 and 350 °C for 20 min. The annealing at 240 °C confirmed the presence of an amorphous phase which crystallized in nanostructured tetragonal SnO phase. The annealing at 350 °C revealed the nucleation of nanostructured tetragonal SnO2 phase.