Published online by Cambridge University Press: 19 September 2016
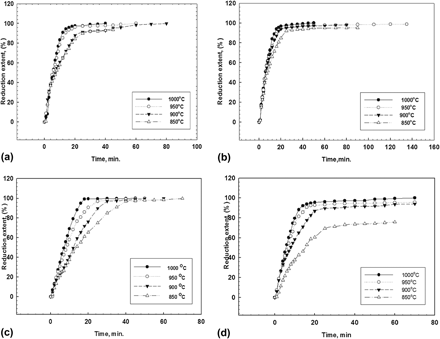
Heavy tungsten alloys with the following compositions 98W2Fe, 93W7Fe, and 95W2Fe3Ni were successfully prepared through gaseous reduction of metal oxide mixtures in the temperature range of 850–1000 °C. Reduced samples were subjected to sintering processes in reducing atmosphere (Ar/4% H2) at different temperatures (1200–1300 °C) and dwell times (30, 90 min). The prepared alloys together with the sintered samples were characterized by x-ray diffraction (XRD), field emission scanning electron microscope (FESEM), and optical microscope. The microhardness of the sintered samples was measured and correlated to sintering temperature and dwell time. The presence of iron oxide decreases the reducibility of WO3 whereas the presence of NiO increases the reducibility of both iron oxide and tungsten oxide. With the increase of sintering temperature and dwell time, porosity of samples decreases forming dense structure which is coupled with the increase of hardness particularly for 95W2Fe3Ni alloy.