Published online by Cambridge University Press: 24 November 2014
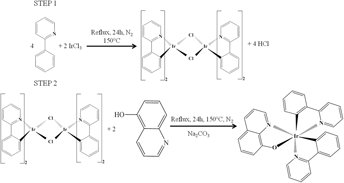
By combining two types of ligands, phenylpyridine and quinoline, a new type of organometallic IrQ(ppy)2 compound has been synthesized, which exhibits two phosphorescences: green and red. Using an appropriate catalyst, the final IrQ(ppy)2 compound has a good chemical yield up to 60% and becomes a stable dual emitter at room temperature. This compound is important because it exhibits stable red emission which is limited by the quantum yield due to the low energy band gap. As a result, an overlap between the ground state and the excited state occurs due to the vibrations that increase the nonradiative transitions, destroying the red emissions. Structural characteristics of the IrQ(ppy)2 powder reveal a triclinic structure confirmed by x-ray diffraction and scanning electron microscopy images. Thermal analysis of the final compound confirms a good stability against decomposition and structural changes up to 350 °C. X-ray photoelectron spectroscopy reveals Ir–O chemical bonds and several differences between the intermediate and final compounds, such as Ir–Cl bonds. Cathodoluminescence patterns show a phosphorescent triclinic structure with a higher efficiency for the red color. Backscattering electron images prove that there is a uniform distribution of iridium ions in the IrQ(ppy)2 nanocrystals.