Article contents
The stabilities and electronic structures of Aln Si12−n N12 (n = 0, 1, 2, and 4)
Published online by Cambridge University Press: 07 January 2016
Abstract
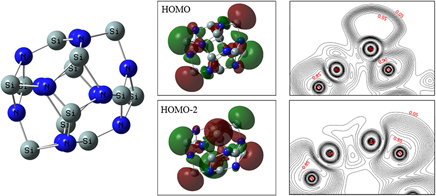
Aln Si12−n N12 (n = 0, 1, 2, and 4) are electron redundant systems. The calculations show that the stabilities of Aln Si12−n N12 and Al12N12 are very close. One Si atom in each Si2N2 square protrudes obviously and the cages are distorted. The excess electrons reside at the outside of the protrudent Si atoms as lone pair electrons. They occupy antibonding orbitals and form the highest occupied band. The Si–N bonds are covalent bonds with strong polarity. The overlap integral is 0.38 per Si–N bond and is 17% stronger than the overlap in Al12N12. The atoms in molecule charge on the in-plane and protrudent Si atoms are 3.13e and 1.65e, respectively. The lone pair electrons form large local dipole moments enhance the electrostatic interaction between the protrudent Si and N atoms. The energy gaps of the electron redundant cages Aln Si12−n N12 (n = 0, 1, 2, and 4) are about 1 eV smaller than the gap of Al12N12. As the lone pair electrons are loosely bond, the SiN-based cages have large hyper-polarizabilities and so have potential applications, such as nonlinear optical materials.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 2
- Cited by


