Published online by Cambridge University Press: 03 March 2011
Epitaxial ZnO thin films were grown on (111) MgAl2O4 with a pre-seeded, two-step chemical solution deposition process. Isolated, epitaxial ZnO islands (seeds) were formed on the substrate in the first step by spin coating a very thin layer of the precursor solution and heat-treating to 950 °C/3 h. In the second step, the seeded substrate was coated with another layer of precursor to produce an epitaxial film. The result was compared with the case in which a MgAl2O4 substrate was not seeded. Both the seeded and unseeded ZnO films have out-of-plane and in-plane orientation relationships of 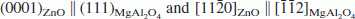 , respectively. However, only the seeded ZnO films have very faceted surface morphology without grain boundaries, indicating epitaxy, whereas the unseeded ZnO films have deep grain boundaries indicative of polycrystalline nature. This result shows that the formation of seeds in the first step plays an instrumental role in the formation of an epitaxial ZnO film.
, respectively. However, only the seeded ZnO films have very faceted surface morphology without grain boundaries, indicating epitaxy, whereas the unseeded ZnO films have deep grain boundaries indicative of polycrystalline nature. This result shows that the formation of seeds in the first step plays an instrumental role in the formation of an epitaxial ZnO film.