Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Saravanan, P.
Jose, T. A.
Thomas, P. John
and
Kulkarni, G. U.
2001.
Submicron particles of Co, Ni and Co-Ni alloys.
Bulletin of Materials Science,
Vol. 24,
Issue. 5,
p.
515.
Choi, Eun Young
Lee, Yoon Bok
Yoon, Suk Young
Kim, Kwang Ho
Kim, Jin Chun
Rhyim, Young Mok
Kim, Hyong Kuk
and
Kim, Yang Do
2005.
Size Control of Nickel Powders from Nickel Chloride Solution Containing Ammonia in DEA Solutions.
Journal of Korean Powder Metallurgy Institute,
Vol. 12,
Issue. 3,
p.
201.
2005.
Preparation of Nickel Powders by the Reduction of Hydrazine from Diethanolamine Solutions.
Journal of the Korean Ceramic Society,
Vol. 42,
Issue. 6,
p.
432.
Chen, Li-Jung
Wan, Chi-Chao
and
Wang, Yung-Yun
2006.
Chemical preparation of Pd nanoparticles in room temperature ethylene glycol system and its application to electroless copper deposition.
Journal of Colloid and Interface Science,
Vol. 297,
Issue. 1,
p.
143.
Elechiguerra, J.L.
Larios-Lopez, L.
and
Jose-Yacaman, M.
2006.
Controlled synthesis of platinum submicron and nanometric particles with novel shapes.
Applied Physics A,
Vol. 84,
Issue. 1-2,
p.
11.
Helmut, Bönnemann
and
Kyatanahalli S., Nagabhushana
2008.
Metal Nanoclusters in Catalysis and Materials Science.
p.
21.
Scharfe, Sandra
Fässler, Thomas F.
Eychmüller, Alexander
Banin, Uri
Dehnen, Stefanie
Eichhöfer, Andreas
Corrigan, John F.
Fuhr, Olaf
Fenske, Dieter
Schmid, Günter
Krylova, Galyna
Bodnarchuk, Maryna I.
Tromsdorf, Ulrich I.
Shevchenko, Elena V.
Talapin, Dmitri V.
and
Weller, Horst
2010.
Nanoparticles.
p.
49.
Sarkar, Anjana
Kapoor, Sudhir
and
Mukherjee, Tulsi
2010.
Synthesis and characterisation of silver nanoparticles in viscous solvents and its transfer into non-polar solvents.
Research on Chemical Intermediates,
Vol. 36,
Issue. 4,
p.
411.
Zhang, Wangqing
Wang, Da
and
Yan, Rui
2011.
Selective Nanocatalysts and Nanoscience.
p.
29.
Nalawade, Pradnya
Mukherjee, Tulsi
and
Kapoor, Sudhir
2012.
High-yield synthesis of multispiked gold nanoparticles: Characterization and catalytic reactions.
Colloids and Surfaces A: Physicochemical and Engineering Aspects,
Vol. 396,
Issue. ,
p.
336.
Abdalhuseen, Rasha Ali
Tummala, S.K.
Kosaraju, S.
Bobba, P.B.
and
Singh, S.K.
2023.
Synthesis of Pd nanoparticle and study the effect on Adenosine amino hydrolase (ADA) enzyme activity in blood serum.
E3S Web of Conferences,
Vol. 391,
Issue. ,
p.
01128.
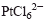 and
and  , respectively, in glycols. Platinum powders contain spherical particles with a bimodal size distribution. Palladium powders also contain spherical particles, but the size distribution is narrow. The effect of both ammonia and hydrazine concentration on the size distribution and average size of palladium particles was investigated.
, respectively, in glycols. Platinum powders contain spherical particles with a bimodal size distribution. Palladium powders also contain spherical particles, but the size distribution is narrow. The effect of both ammonia and hydrazine concentration on the size distribution and average size of palladium particles was investigated.