Published online by Cambridge University Press: 26 April 2016
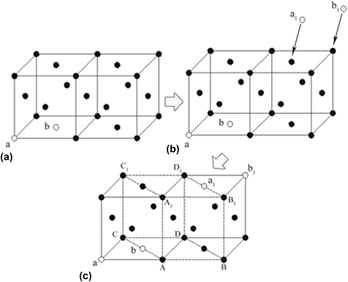
X-ray diffraction analysis, transmission electron microscopy, and thermodynamic calculation were used to investigate the effect of microstructural condition of austenite on the microstructural characteristics of the nanoscale bainite ferrite in a high carbon steel. As austenization temperature increases to 950 °C, there are a higher vacancy concentration and homogenized distribution level of the interstitial carbon atom in the austenite grains. The movement of more di-vacancies combination could encourage the generation of the γ → α embryo nucleus. The interstitial carbon atoms have a stronger inhibitory effect on the formation of the γ → α embryo nucleus and homogenized distribution of the interstitial carbon atoms are able to make the inhibitory effect exist everywhere in the austenite grains. In consequence, the bainite ferrite could only nucleate in a smaller area (several nanometers), and grow into slender laths in a smaller width and a larger length.