Article contents
A relation between the surface energy and the Debye temperature for cubic solids
Published online by Cambridge University Press: 31 January 2011
Abstract
It is shown that a phenomenological relation exists between the Debye temperature θ (in degree Kelvin) and the surface energy Γ (in ergs/cm2) of cubic solids: 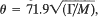 , where M is the atomic weight. This relation is derived theoretically in the Debye isotropic approximation by assuming that the interatomic potential is central. No restrictions are imposed on the range of the potential. The relation is obeyed very well by the observed values of θ and Γ in the case of many solids.
, where M is the atomic weight. This relation is derived theoretically in the Debye isotropic approximation by assuming that the interatomic potential is central. No restrictions are imposed on the range of the potential. The relation is obeyed very well by the observed values of θ and Γ in the case of many solids.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 1990
References
REFERENCES
3Miedema, A. R. and de Chatel, P. F., Theory of Alloy Phase Formation, edited by Bennett, L.H. (TMS-AIME, Warrendale, PA, 1980), p. 344.Google Scholar
6Maradudin, A. A., Montroll, E.W., Weiss, G. H., and Ipatova, I. P., Theory of Lattice Dynamics in the Harmonic Approximation; Solid State Phys. Suppl. (Academic Press, New York, 1971), 2nd ed., Vol. 3.Google Scholar
7Handbook of Materials Science, edited by Lynch, C.T. (C.R.C. Press, Cleveland, OH, 1974).Google Scholar
8Am. Inst. of Phys. Handbook, edited by Gray, D. E. (McGraw-Hill, New York, 1972), 3rd ed.Google Scholar
10Hirth, J. R. and Lothe, J., Theory of Dislocations (Wiley Interscience, New York, 1982), 2nd ed.Google Scholar
- 9
- Cited by


