Published online by Cambridge University Press: 17 November 2014
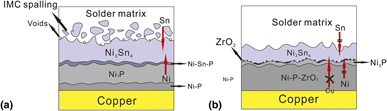
To reinforce the reliability issue brought by excessive interfacial reaction with the dimensional scale-down of electronic device, an electroless Ni–P–ZrO2 (17.5 at.% of P) composite coating was developed as the under bump metallization (UBM) for lead-free solder interconnect. ZrO2 nanoparticles were proved to be homogeneously distributed and helped improve wetting ability of the layer. Both Sn–3.5Ag/Ni–P–ZrO2 and Sn–3.5Ag/Ni–P solder joints were prepared and aged at various conditions to study the interfacial reaction. Growth of intermetallic compounds (IMCs) without serious spalling in solder/Ni–P–ZrO2 joint was slowed down because of the barrier property of incorporation of ZrO2 nanoparticles, which blocked the diffusion of Ni and Cu atoms. Based on the IMC growth, the activation energy of solder/Ni–P–ZrO2 was estimated to be higher than that of plain solder joint. The top-view of IMCs demonstrated a much finer grain size compared with that of solder/Ni–P joint. A reactive diffusion-induced compound formation mechanism was proposed to address the microstructural evolution in detail. Moreover, solder/Ni–P–ZrO2 joint demonstrated higher shear strength than did solder/Ni–P joint for different aging durations. The fracture surface of solder/Ni–P joint after shear test showed ductile transition failure, with big dimples and plastic deformation.