Published online by Cambridge University Press: 24 May 2013
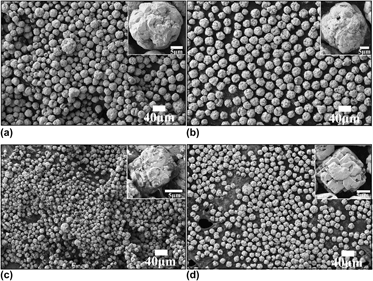
In this work, the morphology of BiFeO3 was successfully modulated from microsphere to microcube by using a polyanion, poly (methyl vinyl ether-alt-maleic acid) (PMVEMA), in a microwave assisted hydrothermal route. A simple ultrasonic purification method has been developed to obtain pure phase BiFeO3 from the crude products without using any chemicals. X-ray diffraction results confirmed the capability of this purification method. When increasing the amount of PMVEMA, the morphology of BiFeO3 gradually changed from microsphere to microcube as illustrated by scanning electron microscopy. A mechanism was suggested for the morphology evolution of BiFeO3. After the formation of the small BiFeO3 single crystal, PMVEMA preferentially absorbed on one side of the crystals through specific and/or noncovalent interactions, resulting in the preferential integration of these crystals to form microcubes. The magnetic properties of these microcrystals were also investigated and the magnetization of the microcubes increased with the decrease of temperature.