Published online by Cambridge University Press: 24 May 2012
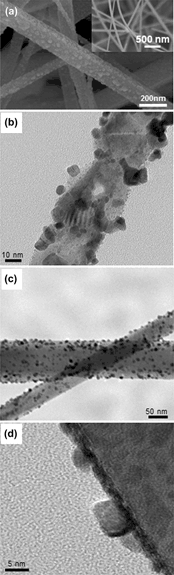
Platinum (Pt) nanoparticles were synthesized on tin dioxide (SnO2) nanowires by applying γ-ray radiolysis. The growth behavior of Pt nanoparticles was systematically investigated as a function of precursor concentration, illumination intensity and exposure time of the γ-rays. We found that these processing parameters greatly influenced the growth behavior of Pt nanoparticles in terms of size and formation density. Vapor-phase-grown SnO2 nanowires were uniformly covered with Pt nanoparticles by the radiolysis process. The Pt nanoparticle-functionalized SnO2 nanowires were tested as sensors for detecting reductive gases including carbon monoxide, toluene, and benzene. The results indicate that the γ-ray radiolysis is an efficient way of functionalizing the surface of oxide nanowires with catalytic Pt nanoparticles.