Article contents
PbO-sensitized ZnO nanorod arrays for enhanced visible-light-driven photoelectrochemical performance
Published online by Cambridge University Press: 02 May 2016
Abstract
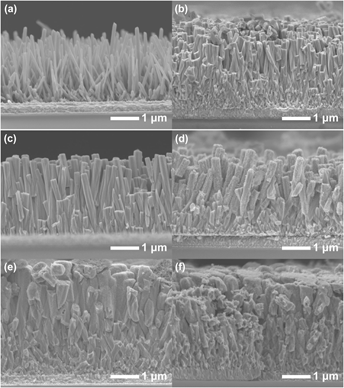
In semiconductor system for solar-energy utilization by photoelectrochemical (PEC) water splitting, the effective absorption of visible light and the efficient separation and transfer of photogenerated charge carriers are still of key importance. In this manuscript, composite photoanodes of PbO sensitized ZnO nanorod arrays were prepared by a two-step hydrothermal process and used as anodes for PEC test under visible-light irradiation. The photocurrent achieved the highest value of 94 μA cm−2 at 0.8 V (versus Ag/AgCl electrode) when the amount of Pb source was optimized to form only a thin layer (a few nanometers) of PbO nanoparticles on the surfaces of ZnO nanorods. Such a nanostructure enabled the visible-light absorption, and also ensured the sufficient contact of PbO with ZnO to form junction with a type II band alignment and the sufficient contact with aqueous solution to form interfaces, thus facilitating the excitation, separation, and transfer of charge carriers to generate photocurrent and finally enhancing the PEC activity.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 31 , Issue 11: Focus Issue: Advanced Materials and Structures for Solar Fuels , 14 June 2016 , pp. 1622 - 1630
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 11
- Cited by



