Published online by Cambridge University Press: 20 May 2019
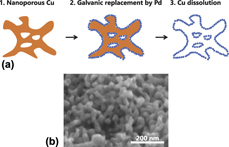
Nanoporous copper (NP-Cu), as a sacrificial support, was used for the synthesis of bimodal nanoporous palladium–copper (BNP-PdCu) for oxygen reduction reaction (ORR) electrodes in fuel cells. The catalytic performance of BNP-PdCu in ORR per electrochemical surface area was enhanced by the dissolution and removal of supporting NP-Cu, which indicates that the intrinsic catalytic properties of palladium are improved by the proposed synthesis strategy including galvanic replacement of copper with palladium, following copper dissolution. Cu remained on Pd surfaces even after dissolution of Cu. Additionally, significant local lattice contraction was observed at the ligament surface. First-principles calculations on the adsorbing oxygen species on Pd show that both lattice contraction and alloying with copper increase the binding energies of oxygen species to the Pd surface. The high ORR activity of the present BNP-PdCu is suggested to be mainly due to the Cu-ligand effect.