Article contents
Optimum temperature range for the proton dynamics in H-doped BaZrO3:Yb dense ceramics—a neutron scattering study
Published online by Cambridge University Press: 13 June 2012
Abstract
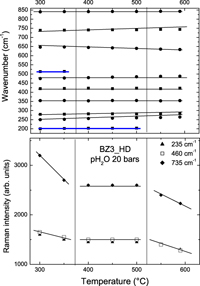
The proton conducting perovskite MZr1−xLnxO3−δHz ceramics are promising electrolytic membranes for fuel cell and water steam electrolyser applications. Simultaneous elastic/quasielastic and diffraction neutron studies were performed in a wide temperature range (25–1150 °C) on protonated Yb-modified BaZrO3 ceramics: dense (97% of theoretical density) and ultradense (99%) using the triple axis spectrometers. The results allowed us to determine: (i) the real content of bulk protonic species ∼1–5 10−3 mol/mol, (ii) the structural modifications caused by the proton doping, and (iii) the bulk proton dynamics. The quasielastic neutron scattering (QNS) results are discussed in the light of neutron diffraction, conductivity, Raman, thermogravimetric, and thermal expansion measurements. The highest bulk proton motion appears in the temperature range where the structural modifications and the energy activation changes are detected. This allows defining the optimum temperature range for the proton dynamics between 400 and 560 °C.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 15: Focus Issue: Advanced Materials for Fuel Cells , 14 August 2012 , pp. 1939 - 1949
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 8
- Cited by


