Article contents
Optical properties of high-performance liquid crystal–xerogel microcomposite electro-optical film
Published online by Cambridge University Press: 21 March 2012
Abstract
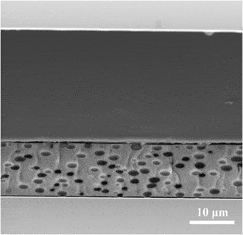
Electro-optical films composed of liquid crystal (LC) microdroplets encapsulated in an organically modified xerogel matrix were prepared by combined sol-gel and phase separation methods. The incorporation of a titanium alkoxide in the synthesis process as a co-precursor to silicon alkoxides was achieved without any destructive influence on the macroscopic LC phase separation. The consequent increase in the refractive index of the matrix satisfied the important criteria for high-performance films. The prepared films exhibit an outstanding 75.9% change in transmittance as an electric field is applied. An original setup was developed that enables the measurement of the film transmittance versus applied voltage at different temperatures over the full visible and near-infrared (near-IR) spectral range. For the first time, the relationship between these important characteristics (i.e., change in transmittance versus wave length versus temperature) was measured for a xerogel-dispersed LC composite film. The maximum of the curve increased and moved from the IR to the midvisible range with increasing temperature, achieving its maximum at 25.2 °C and 551 nm.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 7
- Cited by


