No CrossRef data available.
Published online by Cambridge University Press: 20 August 2020
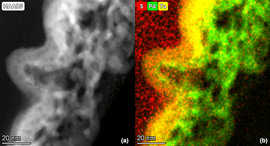
Metallic thin-film composite membranes are produced by sputtering metal films onto commercial polymer membranes. The separations capability of the membrane substrate is enhanced with the addition of a 10 nm Ta film. The addition of a tantalum layer decreases the molecular weight cutoff of the membrane from 70 kDa dextran (19 nm) to below 5 kDa (6 nm). Water flux drops from 168 LMH/bar (LMH: liters/meters2/hour) (polymer support) to 8.8 LMH/bar (Ta composite). A nanoporous layer is also added to the surface through Mg/Pd film deposition and dealloying. The resulting nanoporous Pd is a promising catalyst with a ligament size of 4.1 ± 0.9 nm. The composite membrane's ability to treat water contaminated with chlorinated organic compounds (COCs) is determined. When pressurized with hydrogen gas, the nanoporous Pd composite removes over 70% of PCB-1, a model COC, with one pass. These nanostructured films can be incorporated onto membrane supports enabling diverse reactions and separations.