Published online by Cambridge University Press: 03 July 2012
A quantitative study was made of the composition and microstructure of RuO2 films deposited on three different substrates using reactive sputtering. Most of the films had a composition within 2.5 wt.% of the correct stoichiometry; the only exceptions were films grown on Al2O3 (0001) at 150 °C, which had an oxygen-to-ruthenium ratio of 1: 2.24. The excess oxygen was attributed to a thin oxygen-rich layer that encapsulated the grains. Hydrogen concentrations for the films deposited on Al2O3 (0001) were 14, 6, 6, and < 0.5 at.% for room, 150, 300, and 450 °C growth temperatures respectively. The films deposited at room temperature were amorphous on Al2O3 (0001) and SrTiO3 (100), but weakly crystalline on Al2O3 (1102). Highly oriented RuO2 (100) films were produced on Al2O3 (0001) at deposition temperatures ≥150 °C. The in-plane alignment was 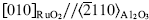 and a threefold mosaic microstructure was observed. The grain boundaries in these films were discontinuous until the substrate temperature was raised to 450 °C, where coherent grain boundaries were formed. The films grown on Al2O3 (1102) at 450 °C exhibited the epitaxial relationship: RuO2(101)//Al2O3 (1102). The in-plane alignment was RuO2〈101〉//Al2O3〈1101〉, and the lattice parameters were the same as found in bulk RuO2. Transmission electron microscopy indicated a large degree of imperfection in the region between coalescing grains. The RuO2 films grown on SrTiO3 (100) at room temperature were amorphous. The film grown at 450 °C showed a preferential orientation with RuO2 (100)//SrTiO3 (100), but without in-plane orientation.
and a threefold mosaic microstructure was observed. The grain boundaries in these films were discontinuous until the substrate temperature was raised to 450 °C, where coherent grain boundaries were formed. The films grown on Al2O3 (1102) at 450 °C exhibited the epitaxial relationship: RuO2(101)//Al2O3 (1102). The in-plane alignment was RuO2〈101〉//Al2O3〈1101〉, and the lattice parameters were the same as found in bulk RuO2. Transmission electron microscopy indicated a large degree of imperfection in the region between coalescing grains. The RuO2 films grown on SrTiO3 (100) at room temperature were amorphous. The film grown at 450 °C showed a preferential orientation with RuO2 (100)//SrTiO3 (100), but without in-plane orientation.