Article contents
Mesoporous titanium dioxide nanobelts: Synthesis, morphology evolution, and photocatalytic properties
Published online by Cambridge University Press: 30 May 2012
Abstract
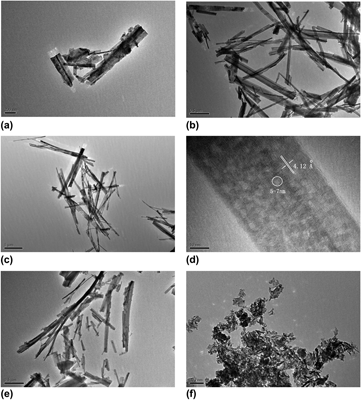
Mesoporous titanium dioxide (TiO2) and lithium (Li)-doped TiO2 nanobelts were synthesized via a facile solvothermal process. The crystalline structure and morphology of the nanobelts were characterized in detail. The x-ray diffraction patterns, transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images indicate that the nanobelts have uniform monoclinic geometry with a length of 3–4 μm and a width of 40–200 nm, the pores are also uniform with 5–7 nm in diameter. scanning electron microscopy and TEM studies demonstrate the as-prepared TiO2 nanobelts have varied morphologies that strongly depend on the volume ratio of the reaction medium and the pressure. Ultraviolet-visible diffuse reflectance spectroscopy was used to study the photocatalytic degradation of Malachite green over the lithium nanoparticle-loaded mesoporous TiO2 nanobelts. The doping of lithium does not change the crystalline phase but the results form infrared spectrums confirm that the Li+ ion incorporates into the lattice of TiO2 nanobelts, decomposes it by replacing Ti4+ and thus reduces the photocatalytic activity.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 17: Focus Issue: Oxide Semiconductors , 14 September 2012 , pp. 2265 - 2270
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 5
- Cited by


