Article contents
Li+ for Na+ ion-exchange-induced phase separation in borosilicate glass
Published online by Cambridge University Press: 28 February 2012
Abstract
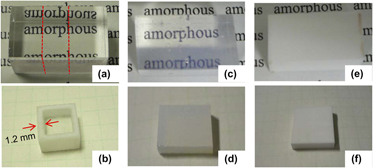
Li+ for Na+ ion-exchange-induced phase separation in borosilicate glass was investigated. A glass with a composition, 70SiO2·15B2O3·15Na2O, was prepared. The glass was transparent, and macroscopically no phase separation was observed because the immiscibility temperature for the composition was lower than the glass transition temperature. Substitution of Li+ for Na+ at the surface domain of the glass induced phase separation in the domain and subsequent heat treatment evolved interconnected silica-rich and alkali borate-rich phases through the phase separation. However, the parts in which ion exchange did not occur kept homogeneous and transparent. This phenomenon is explainable by the fact that the immiscibility temperature for the Li+-substituted composition of the original glass elevated up to a temperature higher than the ion exchange and heat treatment temperatures. The borate-rich phases leached by acid treatment for the phase-separated glass. Through these processes, we obtained monolithic glasses consisting of porous domains, and homogeneous and transparent parts.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 7
- Cited by


