Published online by Cambridge University Press: 07 September 2020
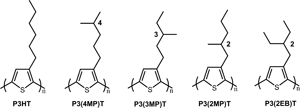
Flexible alkyl side chain in conjugate polymers (CPs) improves the solubility and promotes solution processability, in addition, it affects interchain packing and charge mobilities. Despite the well-known charge mobility and morphology correlation for these semi-crystalline polymers, there is a lack of fundamental understanding of the impact of side chain on their crystallization kinetics. In the present work, isothermal crystallization of five poly(3-alkylthiophene-2,5-diyl) (P3ATs) with different side-chain structures were systematically investigated. To suppress the extremely fast crystallization and trap the sample into amorphous glass, an advanced fast scanning chip calorimetry technique, which is able to quench the sample with few to tens thousands of K/s, was applied. Results show that the crystallization of P3ATs was greatly inhibited after incorporation of branched side chains, as indicated by a dramatic up to six orders of magnitude decrease in the crystallization rate. The suppressed crystallization of P3ATs were correlated with an increased π–π stacking distance due to unfavorable side-chain steric interaction. This work provides a pathway to use side-chain engineering to control the crystallization behavior for CPs, thus to control device performance.
These authors contributed equally.