Published online by Cambridge University Press: 04 July 2016
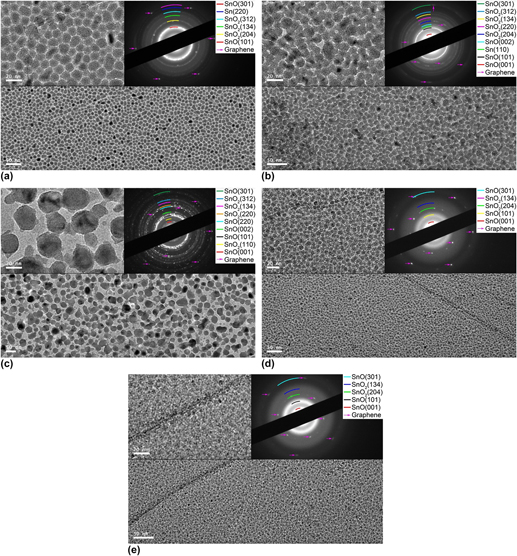
The 1 nm tin oxides–tin (SnO x –Sn) compound films were thermally evaporated onto the chemical vapor deposition (CVD)-grown graphene films for the improved nitrogen dioxide (NO2) gas sensitivity, and the effects of the fabrication temperature and oxygen (O2) flux on the properties of the SnO x –Sn/graphene hybrid sensors including their composition, morphology, and microstructure as well as NO2 sensitivity were investigated. The composition of the SnO x –Sn compound films exhibited strong dependence on the fabrication temperature and O2 flux which could be ascribed to the hybrid effect of the desorption of the oxygen functional groups on the graphene and oxidation of the graphene and Sn. Such combining effects also demonstrated tremendous influence on the SnO x –Sn film morphology, in which the enhanced desorption of the oxygen functional groups on the graphene together with the oxidation of Sn with increasing fabrication temperature would facilitate the formation of large grain-sized and discontinuous films while the increasing O2 flux showed the opposite effects. Meanwhile, the crystallization of the SnO x –Sn compound films was promoted and deteriorated with the increasing temperature and O2 flux, respectively. The SnO x –Sn film morphology played vital role in NO2 gas sensitivity at room temperature, and the mechanism responsible for that was also discussed.
Contributing Editor: Gary Messing