Article contents
Doping properties of hydrogen in ZnO
Published online by Cambridge University Press: 03 July 2012
Abstract
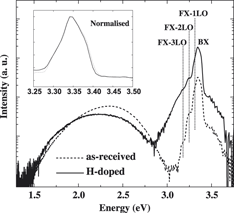
The doping properties and stability of hydrogen in zinc oxide (ZnO) crystals have been investigated by cathodoluminescence (CL) spectroscopy. Hydrogen incorporation was achieved by hydrogen plasma at 200 °C. The ZnO near-band-edge (NBE) peak is dramatically enhanced, while the green emission at 2.4 eV is quenched with increasing hydrogen incorporation. These effects are attributed to hydrogen passivating green luminescence centers, which are most likely negatively charged zinc vacancy defects. E-beam irradiation of H-doped ZnO crystals by an intense electron beam with μW power reverses the hydrogen doping process. This effect is ascribed to the dissociation of H-related defects, formation of “hidden” H2, and electromigration of H+ under the influence of the local trapped charge-induced electric field. These results highlight the potential to modify the local luminescent properties of ZnO by e-beam irradiation.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 17: Focus Issue: Oxide Semiconductors , 14 September 2012 , pp. 2220 - 2224
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 10
- Cited by


