Article contents
Dissociation of water on atomically-defined cobalt oxide nanoislands on Pt(111) and its effect on the adsorption of CO
Published online by Cambridge University Press: 21 January 2019
Abstract
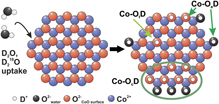
We have investigated the adsorption and dissociation of water and its co-adsorption with CO on atomically defined cobalt oxide nanoislands on Pt(111). The CoO islands were prepared under ultrahigh vacuum (UHV) conditions by reactive deposition of Co metal in oxygen atmosphere. The island structure was characterized by scanning tunneling microscopy (STM), showing that the nanoislands consist of a CoO bilayer and are regularly shaped with island edges that are mainly terminated by Co2+ ions. D2O was dosed in UHV onto the CoO islands on Pt(111) after pre-saturation with CO. D2O dissociation was monitored in situ by isothermal and temperature programmed infrared reflection absorption spectroscopy (IRAS). Isotopic exchange experiments were performed with H2O, D2O, and D218O to elucidate the nature of the hydroxyl groups. Three principal types of OD species are identified: (i) isolated OD at the edges of the CoO islands (Co-OeD), (ii) OD groups within larger hydroxylated areas on the CoO islands (Co-OcD), and (iii) isolated OD groups on the CoO terraces (Co-OtD). At 400 K, water adsorbs dissociatively on the CoO islands and forms isolated hydroxyl species (Co-OeD) at the island edges only. At room temperature (300 K), the coverage of hydroxyl groups increases rapidly, in line with the water-assisted hydroxylation reaction suggested previously. Adsorption experiments with D218O suggest that two equivalent groups are formed from one water molecule after dissociation at island edges, leading to the formation of larger hydroxylated areas on the CoO islands (Co-OcD) and, in addition, isolated OD species on the CoO terraces (Co-OtD). While the initial step of D2O dissociation is facile, the formation of larger hydroxylated areas is a slow and irreversible process. At 200 K, the formation of hydroxylated areas is accompanied by the co-adsorption of molecular water. The hydroxyl groups on the CoO islands are shown to interact with the CO preadsorbed on the CoO/Pt(111) model system. In particular, we observe a new CO species, stabilized by OD groups on the CoO islands, which adsorbs much stronger than CO on the OD-free CoO surface.
- Type
- Invited Article
- Information
- Journal of Materials Research , Volume 34 , Issue 3: Focus Issue: Understanding Water-Oxide Interfaces to Harness New Processes and Technologies , 14 February 2019 , pp. 379 - 393
- Copyright
- Copyright © Materials Research Society 2019
References
- 10
- Cited by


