Article contents
Comparison of structure and electrochemical properties for PANI/TiO2/G and PANI/G composites synthesized by mechanochemical route
Published online by Cambridge University Press: 06 March 2013
Abstract
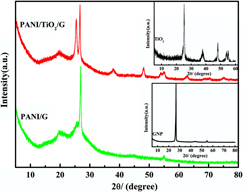
Polyaniline/nano titanium dioxide/graphene nanoplatelet (PANI/TiO2/G) composite was synthesized by mechanochemical route. The structure and morphology of the composite were characterized by Fourier transform infrared spectra, ultraviolet-visible absorption spectra, x-ray diffraction and transmission electron microscopy. The electrochemical performances of the composite were investigated by galvanostatic charge-discharge, cyclic voltammetry, cycling stability and electrochemical impedance spectroscopy. The structure and properties of PANI/TiO2/G composite were compared with that of polyaniline/ graphene nanoplatelet (PANI/G) composite prepared under the same polymerization conditions. After comparative analysis with PANI/G, the effects of the nano titanium dioxide (TiO2) on the structural and physicochemical properties of the PANI/G have been discussed in depth. The comparison suggested that the PANI/TiO2/G composite has higher oxidation degree and lower crystallinity than PANI/G due to the addition of nano-TiO2. Morphology studies showed that PANI and nano-TiO2 particles were both observed on the bent and flat surfaces of graphene nanoplatelet in the PANI/TiO2/G composite. The electrochemical tests showed that the PANI/TiO2/G composite displayed a higher electrochemical activity with specific capacitance of 516 F/g (3 mA/cm2) and better cycle stability than PANI/G.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 9
- Cited by


