Article contents
Biomolecular sensing using gold nanoparticle–coated ZnO nanotetrapods
Published online by Cambridge University Press: 11 August 2011
Abstract
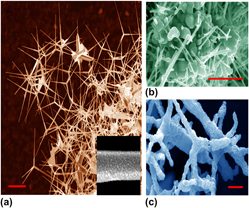
Gold nanoparticle–coated ZnO tetrapods have been utilized as a substrate for the detection of fluorescently labeled protein tetramethylrhodamine isothiocyanate bovine serum albumin and phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(Lissamine rhodamine B sulfonyl) down to the concentrations of 15 pM and 79 nM, respectively. Our detection scheme is based on enhanced fluorescence excitation of the biomolecular analytes by the surface plasmon polaritons of gold nanoparticles coated on the ZnO tetrapod whiskers. This enhanced excitation is confirmed using COMSOL Multiphysics, where the optical near field is shown to be dependent on the coating density of the gold nanoparticles and branching of the ZnO nanostructures.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 26 , Issue 17: Focus Issue: Nanowires: Fundamentals and Applications , 14 September 2011 , pp. 2328 - 2333
- Copyright
- Copyright © Materials Research Society 2011
References
REFERENCES
- 4
- Cited by


