Published online by Cambridge University Press: 17 July 2020
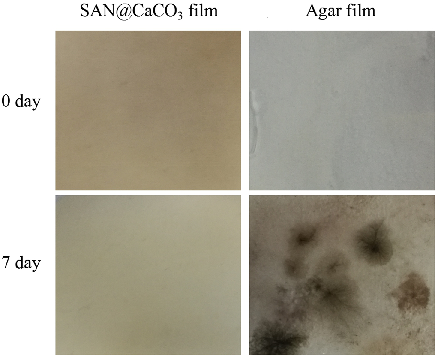
The synthesis of antibacterial biomaterial with specific functions responsive to specific bacterial growth environments is of significant importance to achieve effective sterilization and reduce the resistant bacteria. Herein, inspired by biomineralization, we develop a one-pot, threonine (Thr)-mediated biomineralization method using a CO2 bubbling procedure to green, simply and quickly prepare vaterite CaCO3 microspheres as a platform for antibacterial Sanguinarine (SAN) delivery. The loading capacity of vaterite CaCO3 microspheres for SAN drugs reached 159.8 mg/g, corresponding to the loading efficiency of 83.7%. And for the first time, a novel Sanguinarine@calcium carbonate (SAN@CaCO3) organic–inorganic hybrid antibacterial biofilm was constructed by using vaterite CaCO3 microspheres with pH-responsive and high SAN drug-loading. Importantly, the film showed bacteria-triggered, pH-responsive SAN release properties and strong bactericidal ability (96.19%) for Staphylococcus aureus (S. aureus). Meanwhile, it also had antibacterial capabilities in real environments. In 7 days, it can significantly inhibit the adhesion and growth of bacteria in the air. The biomineralized synthetic vaterite CaCO3 microspheres and the application in the construction of pH-responsive antibacterial biofilm have bright future in resisting bacterial infections and reducing the production of resistant bacteria.