Introduction
A variety of factors have been demonstrated to influence the audiological outcomes and speech perception performance following cochlear implantation. These include age at hearing loss onset, age at implantation, duration of severe to profound hearing loss and level of pre-operative residual hearing.
Intracochlear electrode position has emerged as another potentially significant contributor to outcome. Specifically, the insertion of the electrode array directly to scala tympani, without translocation to scala vestibuli, has been associated with better speech perception outcomes and more successful preservation of residual acoustic hearing.Reference Aschendorff, Kubalek, Turowski, Zanella, Hochmuth and Schumacher1–Reference O'Connell, Hunter and Wanna5 Optimal electric stimulation requires atraumatic electrode insertion, with the electrode contacts within scala tympani for direct spiral ganglion stimulation. The mechanisms that may explain poorer outcomes with scala vestibuli insertion, or scala tympani to scala vestibuli translocation, are: damage to essential neurosensory elements (basilar membrane, organ of Corti and Reissner's membrane), and modiolar proximity variation and cross-turn stimulation, which can lead to pitch confusion.
Successful cochlear implant electrode placement in scala tympani requires that the electrode is inserted through the round window, or through an extended round window or separate cochleostomy, which opens into scala tympani. It is now recognised that an anteriorly placed cochleostomy risks entry into scala media and scala vestibuli. This applies for either straight or pre-curved (peri-modiolar) electrodes. Notably, round window and extended round window approaches have lower rates of placement outside the scala tympani in comparison to a cochleostomy approach, with up to a 70 per cent reduction in scala vestibuli insertion with round window and extended round window approaches.Reference Wanna, Noble, Carlson, Gifford, Dietrich and Haynes4, Reference O'Connell, Hunter and Wanna5 In some cases, scala tympani obstruction with fibrosis or ossification, particularly in advanced otosclerosis or post-meningitis cases, can mandate scala vestibuli placement as the only option for electrode insertion.
Peri-modiolar electrodes such as the Contour Advance™ electrode arrays were designed to decrease electrical stimulation thresholds and provide more targeted electrostimulation.Reference Hughes and Abbas6 It has been demonstrated, however, that for Contour electrodes inserted into scala tympani, there is an incidence of scalar dislocation, with translocation from scala tympani to scala vestibuli usually occurring at the ascending basal turn. Pre-curved electrode arrays such as the Contour Advance are held straight, with an internal platinum stylet to facilitate initial insertion through the cochleostomy or extended round window opening. The pre-curved electrode is then pushed off the stylet and should follow the scala tympani lumen around the modiolus without any lateral wall contact. If, however, the electrode is initially over-inserted, or potentially if orientation is incorrect, then penetration of the basilar membrane can occur, and the electrode translocates and is advanced into scala vestibuli.
Boyer et al. demonstrated that 23 out of 31 peri-modiolar arrays (74 per cent) were placed completely within scala tympani.Reference Boyer, Karkas, Attye, Lefournier, Escude and Schmerber2 This rate was considerably lower in studies by Wanna et al.,Reference Wanna, Noble, Carlson, Gifford, Dietrich and Haynes4 who reported a rate of 58 per cent (40 out of 69 peri-modiolar arrays were completely within scala tympani), and O'Connell et al.,Reference O'Connell, Hunter, Gifford, Rivas, Haynes and Noble7 who reported a rate of 47 per cent (17 out of 36 peri-modiolar arrays were completely within scala tympani). In contrast, the rates for lateral wall arrays were all above 89 per cent. Although all these studies have found significant differences between types of electrodes and approaches, the overall patient numbers are relatively low.
Initial understanding of intracochlear electrode position was based on human cadaveric temporal bone studies. Post-operative clinical studies relied on cochlear view plain X-rays, which enable a good assessment of angular depth of insertion but not of scalar localisation. More recently, a variety of imaging modalities have been used to study intracochlear electrode position in cochlear implant recipients. These include rotational tomography, computed tomography (CT) and cone-beam CT. Post-operative CT images can also be co-registered with pre-operative magnetic resonance imaging (MRI) scans for clearer demonstration of scalar position.
The primary objective of this study was to examine the Melbourne Cochlear Implant Clinic experience with peri-modiolar electrodes, and identify the rates of scala tympani complete insertion, scala tympani and scala vestibuli translocation, and unplanned and planned scala vestibuli insertion. We also wanted to compare the speech perception between groups of patients with different electrode placements. As well as the Cochlear™ Nucleus® Profile Contour Advance Electrode (‘CI512’), we now have experience with the Slim Modiolar Electrode (‘CI532’); hence, we can compare intracochlear position and speech perception outcomes between the two different peri-modiolar arrays.
Materials and methods
A prospective observational study was conducted of 110 peri-modiolar cochlear implantations, involving 92 of the CI512 arrays and 18 of the CI532 arrays. The study was conducted with the approval of the Royal Victorian Eye and Ear Hospital's Human Research Ethics Committee (East Melbourne, Australia). Patients aged over 18 years who underwent cochlear implantation through the Melbourne Cochlear Implant Clinic between January 2015 and March 2016, and who had undergone post-operative cone-beam CT scanning, were included.
Surgical procedure
The surgical approach used was a standard transmastoid facial recess technique. All insertions were performed via separate cochleostomy or extended round window cochleostomy. Operative notes were reviewed particularly to identify cases where intracochlear pathology necessitated deliberate scala vestibuli insertion.
Image analysis
All cone-beam CT images were separately interpreted by an ENT registrar, an otology fellow and a senior otologist. Electrode localisation was categorised into three groups: (1) complete scala tympani placement; (2) complete scala vestibuli placement; and (3) translocation from scala tympani to scala vestibuli. In the majority of cases, electrode position was clear. In 19 cases, the cone-beam CT was more difficult to interpret and there was not consensus among all 3 interpreters.
In these 19 cases, co-registration of the pre-operative MRI and post-operative cone-beam CT was performed, as described elsewhere.Reference Dragovic, Stringer, Campbell, Shaul, O'Leary and Briggs8 In short, the cone-beam CT and MRI scans were imported into the image analysis program Amira™ for semi-automatic registration. Three reference points on each image set were marked in three dimensions to allow automatic multipoint registration. The final registration was performed freehand, to ensure that the cochlea, vestibule and semicircular canals were aligned, with researcher satisfaction determining the final endpoint. This allowed accurate assessment of electrode position for these cases (Figures 1–3).
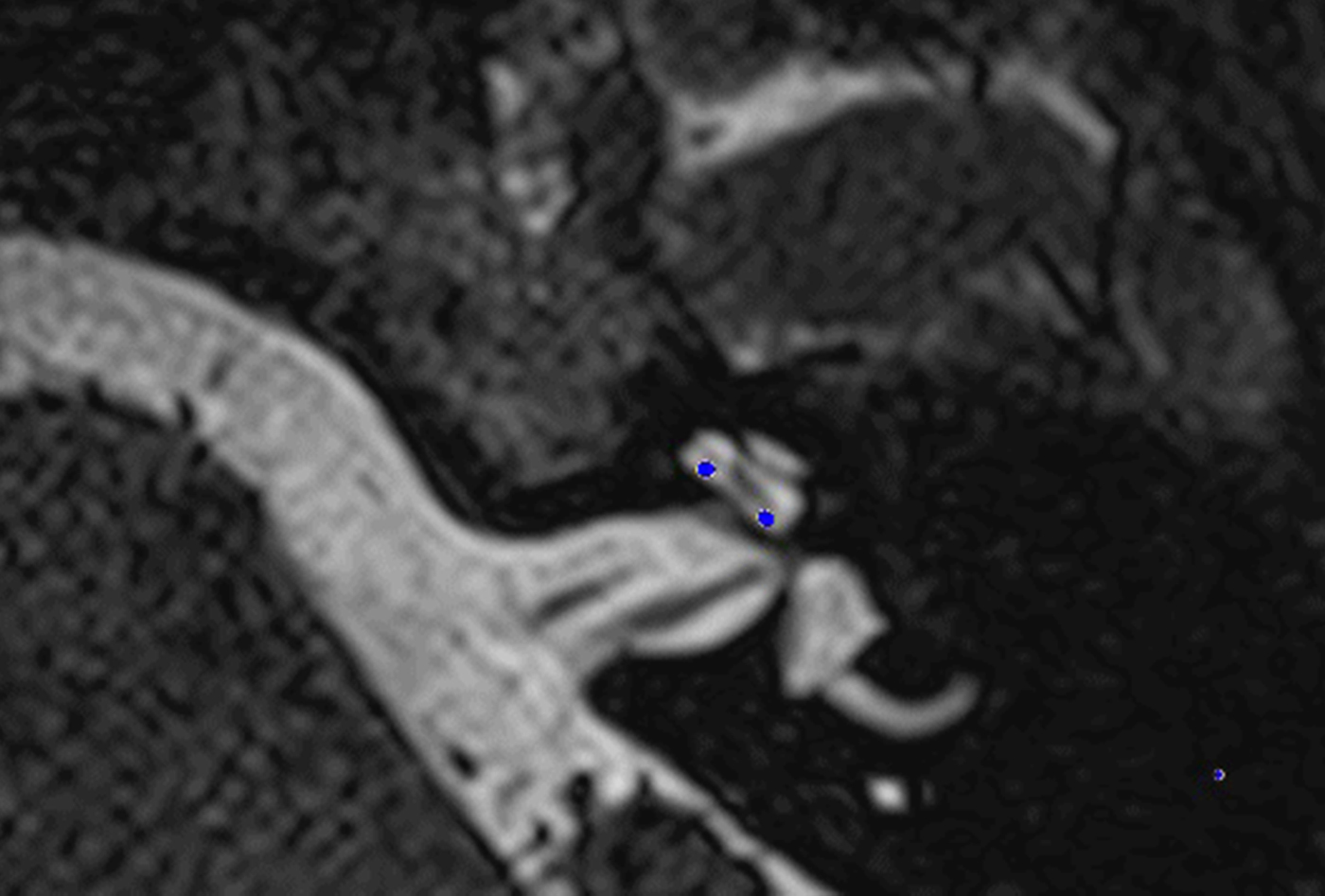
Fig. 1. Co-registered image of pre-operative magnetic resonance imaging and post-operative cone-beam computed tomography scans showing the electrode within scala tympani.
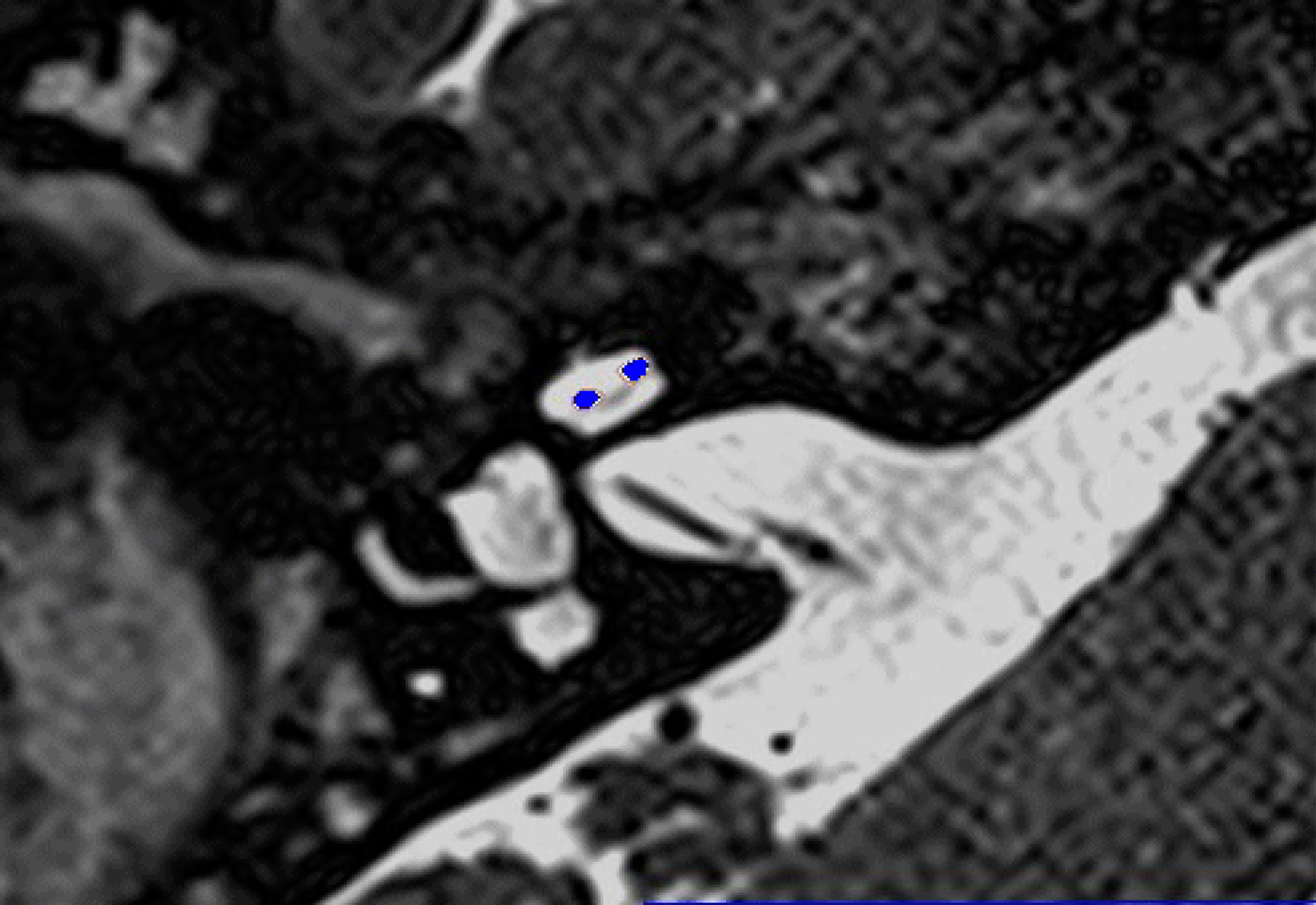
Fig. 2. Co-registered image of pre-operative magnetic resonance imaging and post-operative cone-beam computed tomography scans showing the electrode within scala vestibuli.
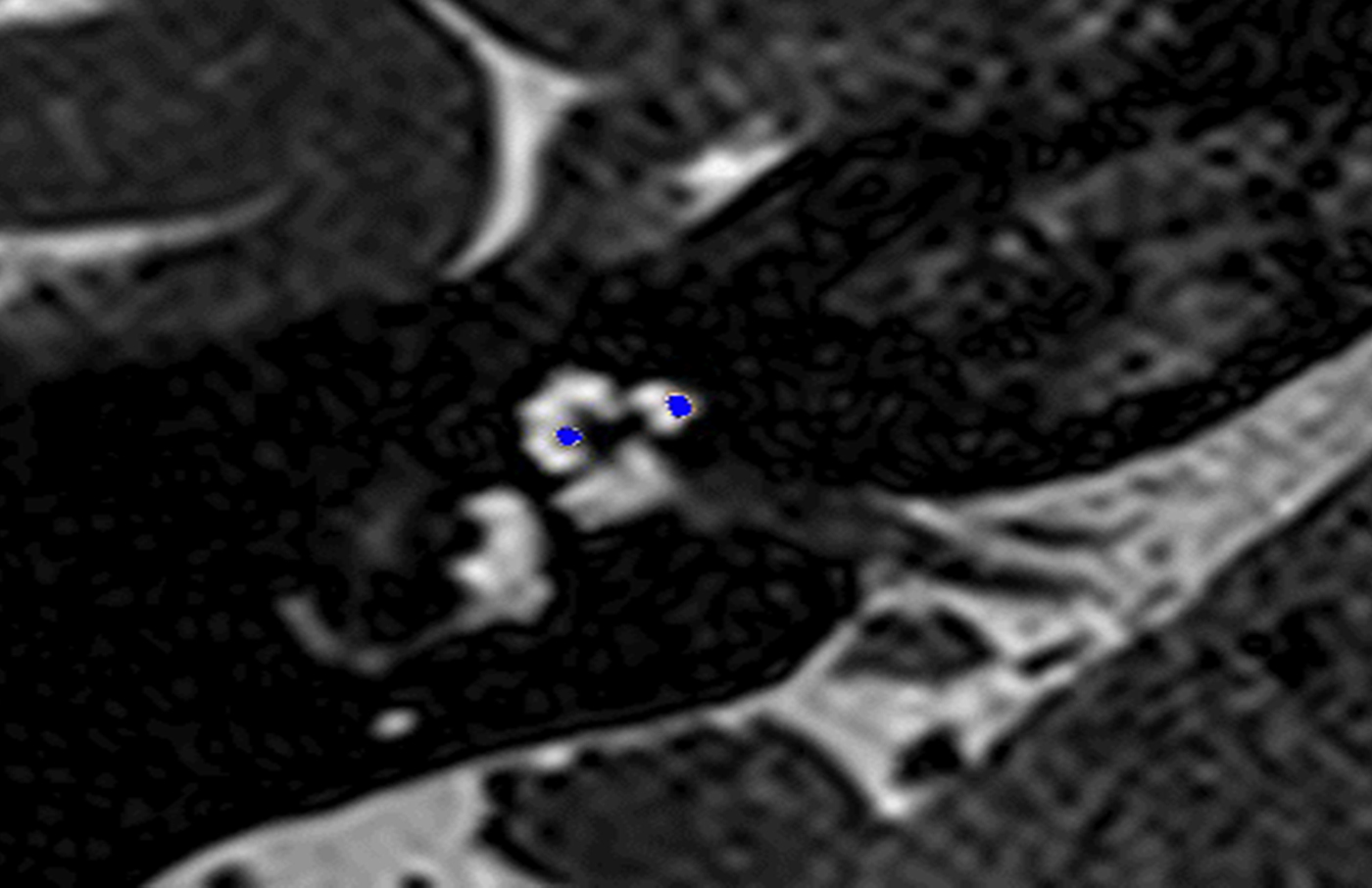
Fig. 3. Co-registered image of pre-operative magnetic resonance imaging and post-operative cone-beam computed tomography scans showing the electrode translocated from scala tympani to scala vestibuli.
Audiology history and performance
Demographic data, including hearing loss aetiology, pre- or post-lingual onset, duration of severe to profound hearing loss before implantation, and pre-operative audiology evaluation findings, were recorded for all patients.
Speech perception testing was repeated 3 and 12 months post-operatively. Participants were required to listen to a list of 50 consonant–vowel–consonant words, which contained 150 phonemes, presented in quiet audition alone, via a loudspeaker, at a level of 65 dB SPL. The participants were required to repeat the word they heard and an audiologist scored the number of phonemes repeated correctly. The consonant–vowel–consonant words were recorded by a male Australian talker, according to the rules stipulated to ensure a balanced list of phonemes.Reference Peterson and Lehiste9
Statistical analysis
Statistical differences in subjects’ demographics and audiology results were evaluated (with IBM SPSS Statistics version 24; Chicago, Illinois, USA) using a two-tailed, two-sample t-test, Mann–Whitney test, analysis of variance or Kruskal–Wallis test, as appropriate, followed by correction for multiple comparisons (Tukey or Bonferroni–Dunn post-hoc tests). The correlations between the individual's demographic details and scala placements and the audiology results were evaluated using a Pearson correlation test. Variables that were found to be associated with audiology performance were entered into a multiple regression model using the stepwise method. Data are presented as means and standard deviations. Statistical significance was defined as p < 0.05.
Results
Surgical procedure performed
All CI532 electrodes and 79 of the CI512 electrodes (86 per cent) were intended to be inserted into scala tympani, while 13 of the CI512 electrodes (14 per cent) were deliberately inserted into scala vestibuli because of anatomical changes (9 patients had far-advanced otosclerosis and 4 had post-meningitis cochlear ossification).
Image analysis findings
Imaging demonstrated that all 18 of the CI532 electrodes were localised in scala tympani. Of the 79 cases in which the CI512 electrode was intended to be inserted into scala tympani, 58 electrodes (73 per cent) were localised in scala tympani, while 14 (17 per cent) were translocated and 7 (9 per cent) were localised in scala vestibuli. Of the 13 cases of deliberate scala vestibuli insertion, 9 electrodes (69 per cent) were wholly in scala vestibuli, and 4 (31 per cent) commenced in scala vestibuli and then translocated to scala tympani (Table 1 and Figure 4).

Fig. 4. Scalar localisation of CI512 electrodes based on intended insertions and imaging localisation. ST = scala tympani; SV = scala vestibuli
Table 1. Translocation of CI512 electrode from scala tympani to scala vestibuli

Data represent numbers (and percentages) of arrays
Audiology history and performance findings
There were no significant differences between the various array localisation groups in regard to: age at implant, duration of severe to profound hearing loss before implantation, percentage of pre-lingual patients, and pre-operative phonemes scores. However, while not significant, the proportion of pre-lingual patients in the scala tympani group (n = 17) was higher than that in the scala vestibuli group (n = 1) and translocation group (n = 4) (Table 2).
Table 2. Comparison between CI512 electrode localisation groups with intended scala tympani insertion

* Analyses conducted using the Kruskal–Wallis test. SD = standard deviation; pre-op = pre-operative; mth = month; post-op = post-operative
Comparison of post-operative phonemes scores between the CI512 electrode localisation groups revealed better scores for scala tympani localisation, at 3 and 12 months post-implantation, but without statistical significance (Table 2 and Figure 5). When the pre-lingual patients were excluded and the post-lingual patients analysed, there were significant differences between the scala tympani group and the other two groups (scala vestibuli and translocation) (p = 0.013). These differences were significant at 12 months post-implantation. Post-hoc analysis demonstrated significant differences between scala tympani and scala vestibuli groups (69.1 per cent vs 54.2 per cent; p < 0.05) and between scala tympani and translocation groups (69.1 per cent vs 50 per cent; p < 0.005) at 12 months post-implantation, but without significant differences between scala vestibuli and translocation groups (Table 3 and Figure 6).
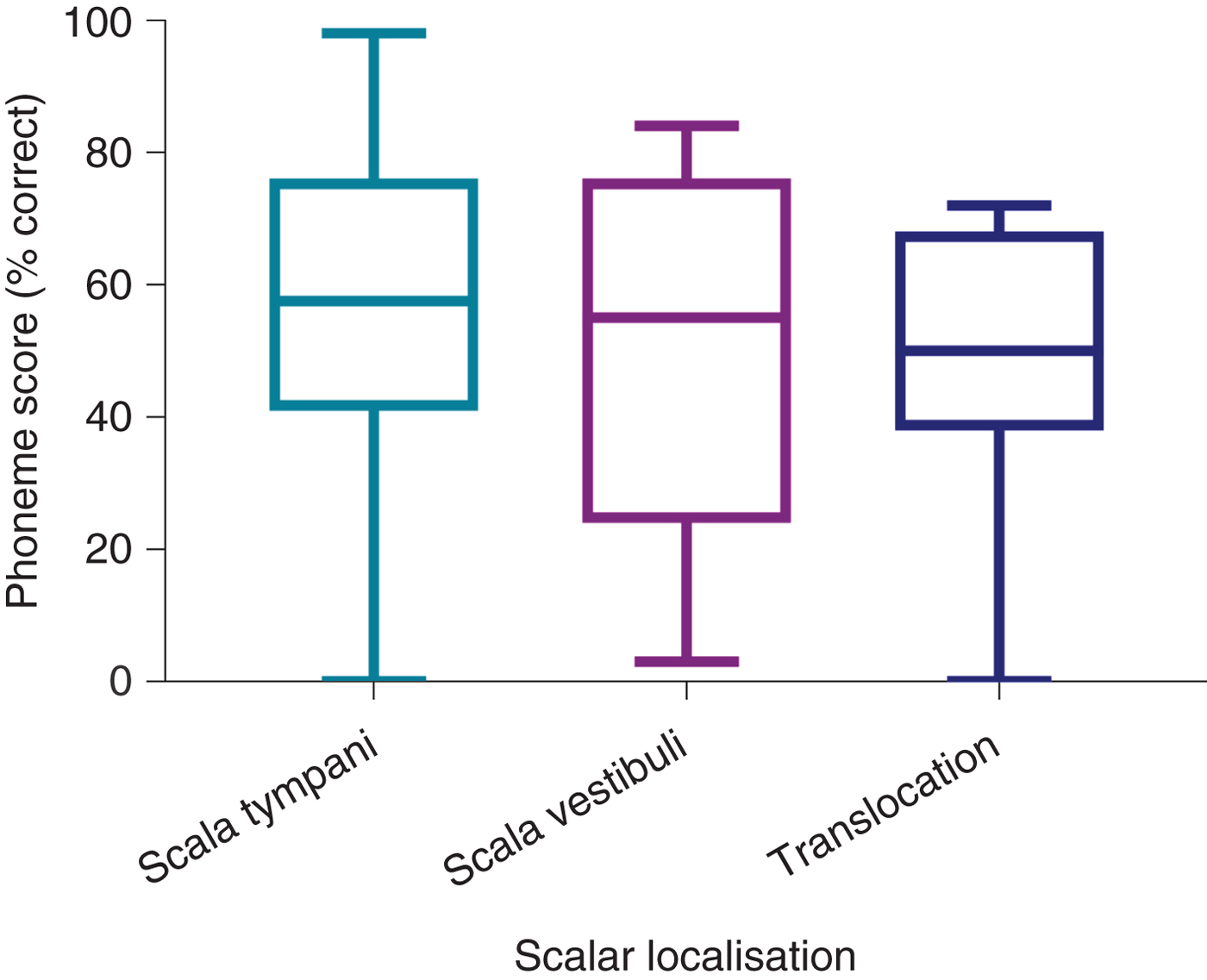
Fig. 5. Twelve-month post-implantation phonemes scores of all patients with CI512 electrodes according to scalar localisation.
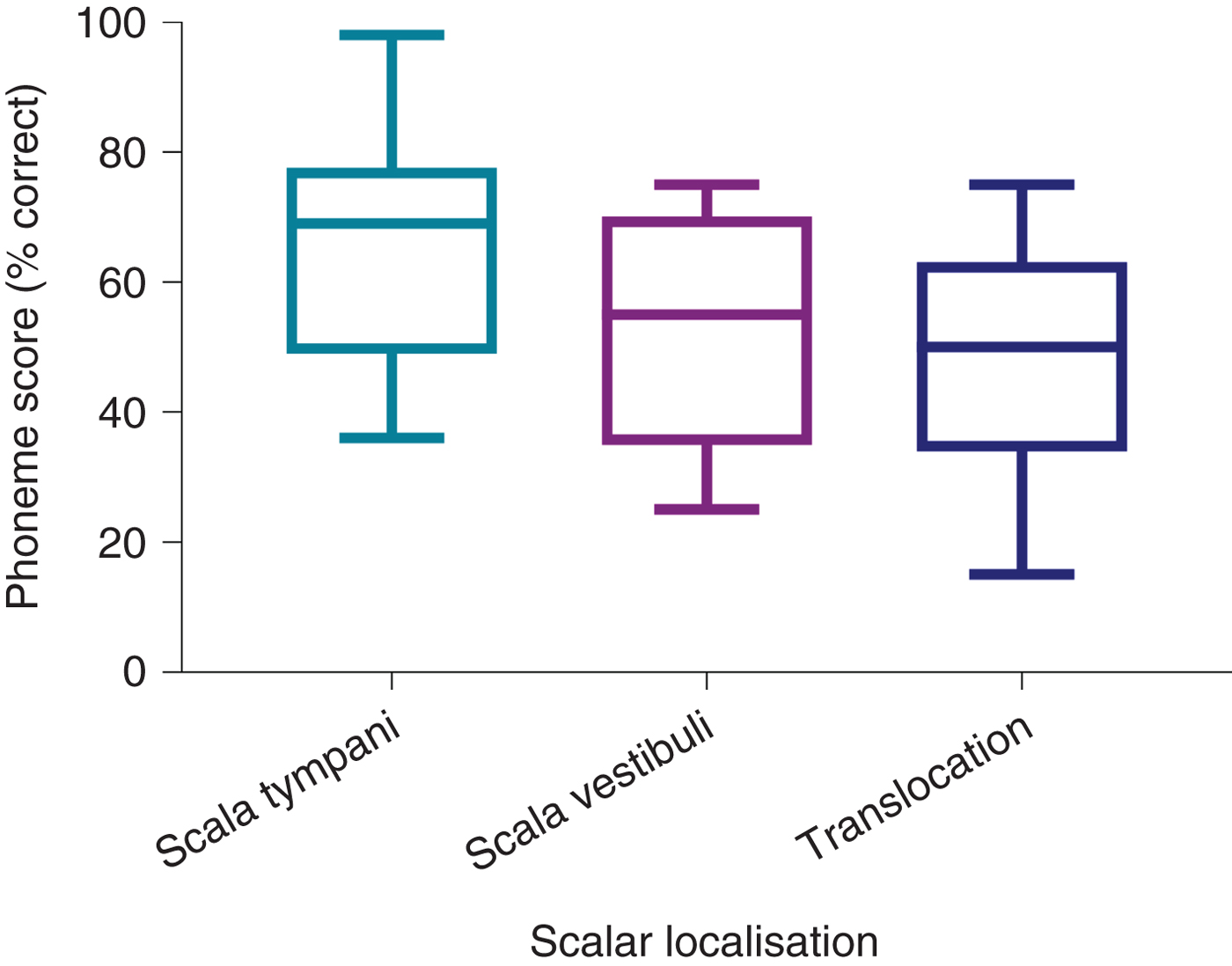
Fig. 6. Twelve-month post-implantation phonemes scores of post-lingual patients with CI512 electrodes according to scalar localisation.
Table 3. Comparison between localisation of CI512 electrodes intended for scala tympani insertion in post-lingual patients

* Analyses conducted using the Kruskal–Wallis test.
† p < 0.05. SD = standard deviation; pre-op = pre-operative; mth = month; post-op = post-operative
In addition, as would be expected, age at implantation and duration of deafness were significantly correlated with 12-month post-operation phoneme scores, with Pearson correlation values of −0.24 and −0.35, respectively. However, pre-implantation phoneme scores were not found to correlate with post-implantation phoneme scores. Therefore, scalar localisation, age at implantation and duration of deafness were entered into a multiple regression analysis model. Those three independent variables were retained in the model, accounting for 25 per cent of the overall variability in the 12-month post-operation phoneme scores. The scores decreased by 0.29 per cent and 0.35 per cent for every one-year increase in age and duration of deafness, respectively (p < 0.01). Electrodes located out of scala tympani demonstrated a 10.5 per cent decrease in phonemes scores (p < 0.05).
For those recipients with planned scala vestibuli electrode insertion because of scala tympani obstruction, the mean 12-month phoneme score was 46.1 per cent (Table 4).
Table 4. Comparison between CI512 electrode localisation groups with intended scala vestibuli insertion

* Analyses conducted using the t-test or Mann–Whitney U test. SD = standard deviation; pre-op = pre-operative; mth = month; post-op = post-operative
CI512 versus CI532
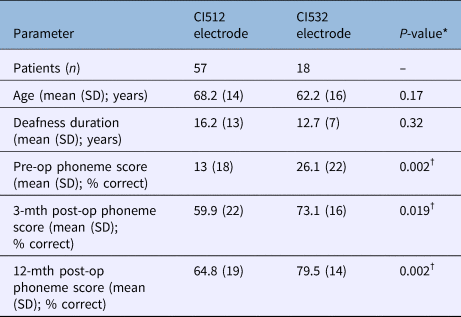
All patients with CI532 electrodes had post-lingual onset hearing loss. The CI532 electrode outcomes were therefore only compared to post-lingual CI512 electrode patients. Phoneme scores at 3 and 12 months post-implantation were higher in the CI532 electrode group (65 per cent) compared to the CI512 electrode group (79 per cent) (Table 5). Furthermore, when compared to the CI512 electrode patients with complete scala tympani insertion, the CI532 electrode group demonstrated better performance. However, phoneme scores were higher pre-operatively for the CI532 electrode group than for the CI512 electrode group (Table 5). Therefore, in order to match the CI512 and CI532 electrode groups, the patients in the CI512 electrode group with lower quartile pre-operative phoneme scores were removed. After matching the groups, the differences become smaller, but remained significant 12 months post-implantation (69 per cent vs 80 per cent) (Table 6 and Figure 7).
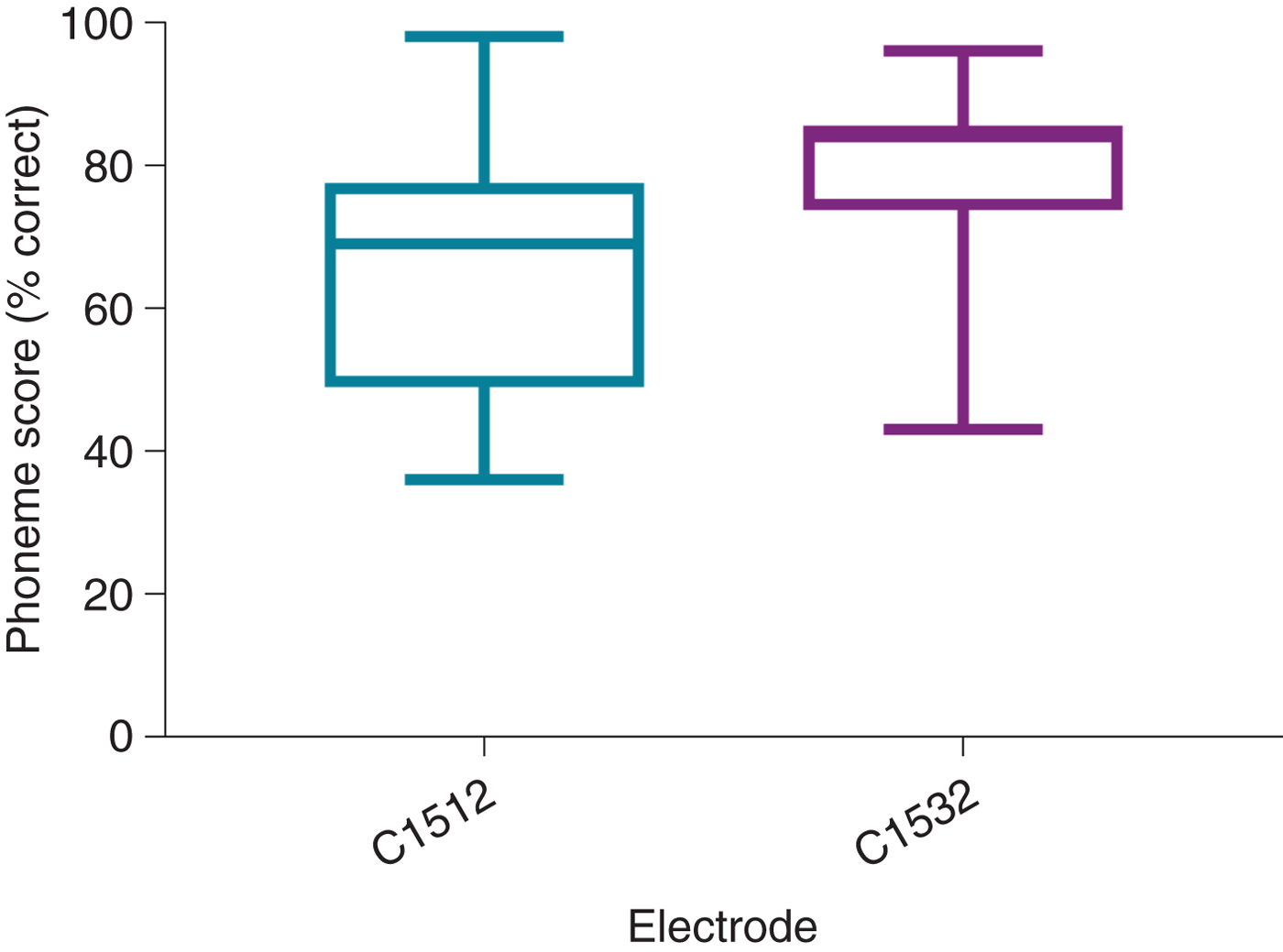
Fig. 7. Twelve-month post-implantation phonemes scores of post-lingual patients with CI512 or CI532 electrodes. All electrodes were in scala tympani. The groups were matched by removing the patients with CI512 electrodes who had lower quartile pre-operative phoneme scores.
Table 5. Comparison between post-lingual patients with CI512 electrodes (with intended scala tympani insertion) and CI532 electrodes
* Analyses conducted using the Mann–Whitney U test.
† p < 0.05. SD = standard deviation; pre-op = pre-operative; mth = month; post-op = post-operative
Table 6. Comparison between matched post-lingual patients with CI512 or CI532 electrodes (with all electrodes localised in scala tympani)

The groups were matched by removing the CI512 electrode group patients with lower quartile pre-operative phoneme scores.
* Analyses conducted using the Mann–Whitney U test.
† p < 0.05. SD = standard deviation; pre-op = pre-operative; mth = month; post-op = post-operative
Discussion
Atraumatic scala tympani insertion that preserves intracochlear structure is the ultimate goal of cochlear implantation. This study used cone-beam CT and, when necessary for clarification, co-registration with pre-operative MRI, to examine peri-modiolar electrode position. The results confirmed previous study findings which showed that, with the CI512 electrode, even when there is intended scala tympani insertion, there is a significant incidence of either direct scala vestibuli placement or translocation from scala tympani to scala vestibuli.
The proportion of complete scala tympani insertion among CI512 electrodes in our study was 73 per cent. This is similar to the finding of Boyer et al.Reference Boyer, Karkas, Attye, Lefournier, Escude and Schmerber2 (74 per cent), and higher than the rates reported by Wanna et al.Reference Wanna, Noble, Carlson, Gifford, Dietrich and Haynes4 and O'Connell et al.Reference O'Connell, Cakir, Hunter, Francis, Noble and Labadie10 (under 50 per cent). Boyer et al.Reference Boyer, Karkas, Attye, Lefournier, Escude and Schmerber2 used a round window approach, while Wanna et al.Reference Wanna, Noble, Carlson, Gifford, Dietrich and Haynes4 and O'Connell et al.Reference O'Connell, Cakir, Hunter, Francis, Noble and Labadie10 implanted using a round window, extended round window or cochleostomy approach. They had the lowest rate of complete scala tympani insertions for peri-modiolar implants with the latter of these approaches (25.9 per cent).
When cochlear anatomy is normal, we believe that the occurrence of scala vestibuli placement or translocation is directly related to surgical technique. A cochleostomy located too anteriorly, or made too large, may predispose the patient to scala vestibuli placement. Translocation occurs because of incorrect use of the Advance Off-Stylet insertion technique. In this study, eight different surgeons operated on the patients, with some having a very low rate of translocation and others a much higher rate. Those surgeons with a high rate of translocation have since been able to modify their insertion technique, ensuring correct electrode orientation and not inserting the array beyond the white mark before Advance Off-Stylet. This has dramatically improved their rates of accurate scala tympani placement.
The CI532 device was introduced during the study period. Although the numbers are small, we felt it worthwhile to include CI532 electrode recipients and to compare their results to CI512 electrode recipients. Of the CI532 electrodes, 100 per cent were shown to be completely in scala tympani. An extended round window approach was used and this clearly provides accurate scala tympani entry. The absence of any translocation with the CI532 electrode is consistent with previously reported temporal bone studies,Reference Briggs, Tykocinski, Lazsig, Aschendorff, Lenarz and Stover11 and is better than the findings reported by McJunkin et al.Reference McJunkin, Durakovic, Herzog and Buchman12 Presumably, insertion without an internal stylet, and the reduced bulk and stiffness of the electrode, can reduce translocation between scala and minimise intracochlear structure damage.Reference Aschendorff, Briggs, Brademann, Helbig, Hornung and Lenarz13, Reference Cuda and Murri14
Post-operative imaging with accurate assessment of intracochlear electrode position is certainly useful, and allows surgeons to validate or modify their surgical technique. A number of imaging modalities have been used to assess electrode location. These include high-resolution CT,Reference Wanna, Noble, Carlson, Gifford, Dietrich and Haynes4 rotational tomography,Reference Aschendorff, Kromeier, Klenzner and Laszig15 flat panel CTReference Arweiler-Harbeck, Monninghoff, Greve, Hoffmann, Goricke and Arnolds16 and cone-beam CT.Reference Boyer, Karkas, Attye, Lefournier, Escude and Schmerber2 The clear advantage of cone-beam CT is the reduced radiation to recipients. The resolution and information that can be obtained from cone-beam CT has improved over recent years and is now superior to routine CT.
In this study, the same cone-beam CT system was used for all patients (i-CAT Next Generation (17–19) cone-beam CT (i-CAT, Hatfield, Pennsylvania, USA)). Most of the electrodes could be accurately localised using this system. However, in 19 cases, we used co-registration with pre-operative MRI studies in order to obtain better information. Recently, we have used a new system, the NewTom 5 G XL cone-beam CT system (Verona, Italy). This provides dramatically better resolution, with no need for co-registration with pre-operative MRI for electrode localisation. The routine in the Melbourne Cochlear Implant clinic is to perform a temporal bone cone-beam CT for every adult recipient during the early post-operative period. This has replaced the previously used cochlear view plain X-ray (modified Stenvers view). Cone-beam CT allows accurate localisation of all electrodes within the cochlea, and is useful not only for research purposes but also to identify the unusual case of tip fold-over and need for re-implantation.
In 13 cases, there was intracochlear fibrosis or ossification obstructing scala tympani in the basal turn, and so it was necessary to insert the electrodes directly into scala vestibuli. In nine of these cases, cone-beam CT confirmed that the electrodes were located in the scala vestibuli; however, in four cases, translocation was observed from scala vestibuli to scala tympani. The mean 12-month phoneme score for these planned scala vestibuli recipients was 46.1 per cent. It is reassuring to see that these recipients obtained significant benefit with their implants, despite the degree of intracochlear disease. Their performance is significantly worse than the post-lingual patients with scala tympani placement. This is presumably in part a result of the degree of cochlear disease rather than the electrode position. However, the number of patients in this group with scala vestibuli to scala tympani translocation is too small to reach any statistical significance.
The degree to which scalar localisation contributes to the speech recognition outcome is the most relevant endpoint for clinicians. Our findings demonstrated that intracochlear electrode placement has a significant influence on the variability of post-implantation speech recognition results. There was a trend toward better consonant–vowel–consonant word scores for the complete scala tympani insertion group of combined pre- and post-lingual recipients, compared to the scala vestibuli and translocation groups, but this did not reach significance. Analysis of the scala tympani group revealed a much higher proportion of pre-lingual recipients, which may explain the poorer results for that group. Previous studies examining scalar localisation have reported outcomes for post-lingual recipients only.
Given that poorer outcomes are expected in recipients with a pre-lingual component to their hearing loss, a further analysis that included only post-lingual recipients was performed, in order to minimise the variables. This comparison demonstrated that the scala tympani group speech performance scores were higher by 15 per cent on average, compared to the scala vestibuli group. Moreover, according to the regression analysis model, electrodes located in scala vestibuli, or translocated from scala tympani to scala vestibuli, independently reduced the 12-month post-implantation phoneme scores by 10.5 per cent, while no significant difference was found between scala vestibuli and translocation localisation.
• Cone-beam computed tomography can demonstrate post-operative scalar localisation of intracochlear peri-modiolar electrodes
• The rate of complete insertion in scala tympani was higher in ‘CI532’ (100 per cent) than in ‘CI512’ (73 per cent) electrode patients
• Electrodes not located fully in scala tympani were associated with 10 per cent lower speech perception post-implantation phoneme scores
These results are consistent with previous studies. Aschendorff et al. were the first to report better speech performance for recipients with scala tympani localisation, poorer performance for those with translocation and poorest results following scala vestibuli insertion.Reference Aschendorff, Kromeier, Klenzner and Laszig15 Skinner et al. reported a significant negative relationship between word recognition (consonant–vowel–consonant word score) and the number of electrodes in the scala vestibuli.Reference Skinner, Holden, Whiting, Voie, Brunsden and Neely17 Scalar position was found to be the most significant contributor (R2 = 0.49) in the consonant–vowel–consonant word recognition model described by Finley et al.Reference Finley, Holden, Holden, Whiting, Chole and Neely3 O'Connell et al. found that consonant–vowel–consonant word scores for complete scala tympani insertion were 51 per cent, as opposed to 39 per cent for scala vestibuli insertion.Reference O'Connell, Cakir, Hunter, Francis, Noble and Labadie10
In this study, all CI532 electrodes were located in scala tympani, as reported by Aschendorff et al. in the initial clinical trial.Reference Aschendorff, Briggs, Brademann, Helbig, Hornung and Lenarz13 McJunkin et al. reported that only 74 per cent (17 out of 23) of CI532 electrode recipients had full scala tympani insertion, however.Reference McJunkin, Durakovic, Herzog and Buchman12 This study is the first to report 12-month post-operative consonant–vowel–consonant word results for CI532 electrode recipients. These recipients achieved significantly better consonant–vowel–consonant word results compared to CI512 electrode recipients. This difference was not only demonstrated in a comparison with the overall group of CI512 electrode recipients intended to have scala tympani insertion but also when compared to the group with confirmed scala tympani localisation. Furthermore, in order to better match the CI512 and CI532 electrode groups, those patients with CI512 electrodes who had lower quartile pre-operative consonant–vowel–consonant word scores were removed from the comparison, and the speech performance of the CI532 electrode group remained better. This study included a low number of CI532 electrode recipients, however, and so it is difficult to draw any definite conclusions regarding comparative outcomes.
Conclusion
This study confirms the utility of cone-beam CT in demonstrating the post-operative scalar localisation of intracochlear peri-modiolar electrodes. Regression analysis confirmed the results of prior studies showing that scala vestibuli insertion, or scala tympani to scala vestibuli translocation, is associated with significantly poorer speech perception outcomes.
Competing interests
None declared















