Introduction
Management of a compromised airway due to tracheal stenosis is notoriously difficult. In the acute setting, especially in inexperienced hands, this can lead to an emergency tracheostomy in the awake patient. In patients with severe stridor, the multidisciplinary team often do not have the luxury of obtaining a computed tomography (CT) scan of the neck and chest, to assess the degree, length and position of the stenosis. However, knowledge of these dimensions is useful before any intervention, to aid decisions regarding the best way to protect the airway without further harming the trachea. Therefore, the most senior anaesthesiologist and otolaryngologist or cardiothoracic surgeon available should be consulted to manage these patients.
Traditional dilatation devices have many shortcomings. Rigid bougies can cause further trauma to the airway, and the tip of the bougie is not visible during dilatation, risking a perforation. Conventional balloons completely occlude the tracheal lumen, making ventilation impossible, adding the risk of hypoxic injury to an already respiratory compromised patient.Reference Achkar, Dowdal, Fink, Franco and Song1 Many such occlusive balloons do not fit through a rigid bronchoscope, and one must rely on suspension laryngoscopy to insert the device, making ventilation more challenging and wasting valuable time during set-up. Dilatation using a rigid bronchoscope causes additional trauma, with the risk of tracheal rupture in inexperienced hands.
In our centre, it is currently standard practice to use a supraglottic airway device and a non-occlusive balloon in all patients who present with tracheal or bronchial stenosis requiring urgent surgical intervention.Reference Hofmeyr, McGuire, Douglas-Jones, Proxenos, Park and Lubbe2–Reference Hofmeyr, McGuire, Park, Proxenos, Peer and Lehmann6 We describe a novel technique for dilatation of stenosis that involves a non-occlusive tracheal dilatation balloon through a supraglottic airway device, performed under direct endoscopic vision.
Technical description
Adult patients presenting or referred to our tertiary academic hospital with tracheal or bronchial stenosis are managed by a special interest multidisciplinary team that includes practitioners from the departments of anaesthesiology, otolaryngology and cardiothoracic surgery. Where possible, patients are admitted to the otolaryngology or thoracic surgery ward for pre-operative assessment, which includes CT imaging of the head, neck and chest. Patients are then promptly scheduled for endoscopy under anaesthesia. If presenting in extremis with acute severe airway compromise, patients are urgently transferred to the operating theatre, where pre-operative endoscopic or ultrasound assessment of the airway may be performed.Reference Hofmeyr, Llewellyn and Fagan7
Anaesthesia preparation and considerations
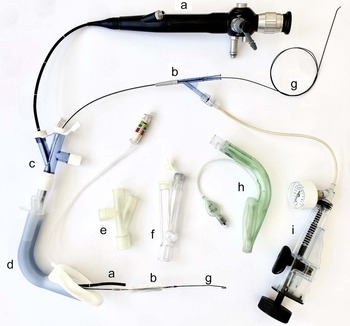
Whenever possible, a two-person anaesthesia team plus an anaesthesia nurse or assistant is assembled within an operating theatre that allows 360-degree access to the patient. The presence of emergency drugs, adjuncts for airway management, a manual jet ventilator and rigid bronchoscopy equipment is confirmed. Furthermore, we employ an airway stack with inter-operable video laryngoscopes, and flexible and rigid video endoscopes, and ensure the availability of a variety of second-generation supraglottic airway devices (local preference is for the Ambu® AuraGain™ or laryngeal mask airway LMA® Protector™). An extra but important adjunct is a multiport airway adaptor obtained from a bronchial blocker set, which allows simultaneous placement of the flexible endoscope, balloon and continuous ventilation (Figure 1).
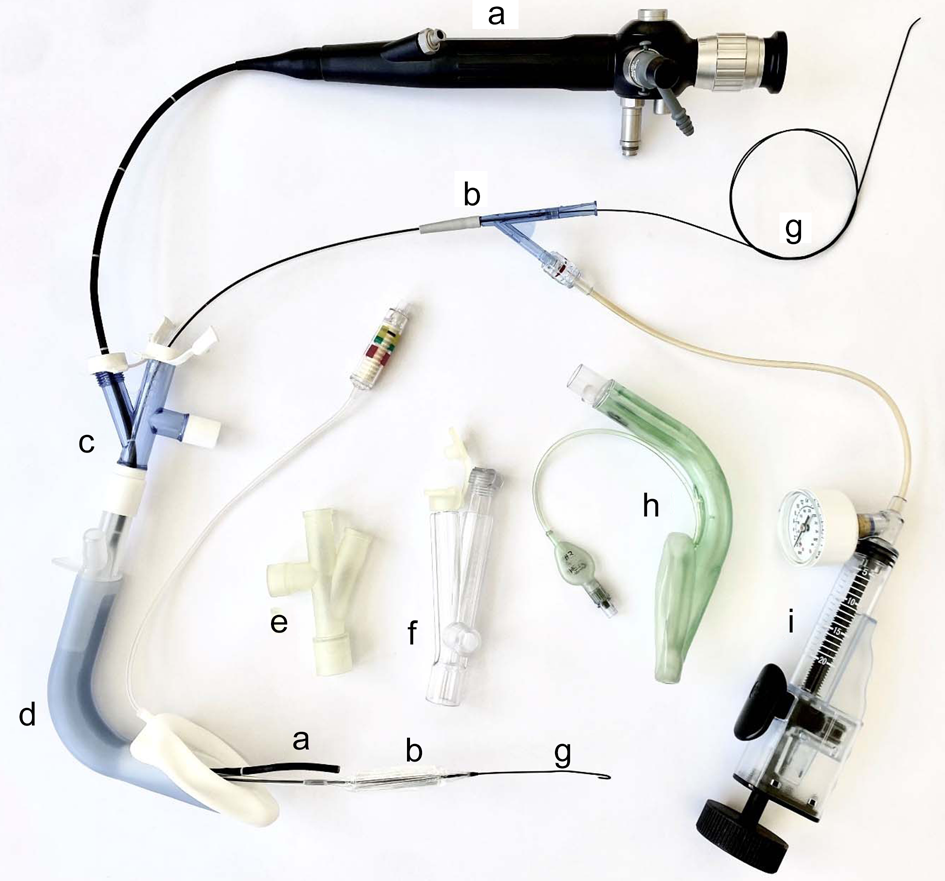
Fig. 1. Equipment used for endoscopic technique through a supraglottic airway device. a = flexible bronchoscope; b = non-occlusive balloon; c = multiport adaptor (Arndt; Cook® Medical); d = supraglottic airway device (laryngeal mask airway LMA® Protector™); e = multiport adaptor (experimental); f = multiport adaptor (Rüsch®; Teleflex®); g = guide wire; h = supraglottic airway device (Ambu® AuraGain™); i = pressure insufflator
Intravenous access is established using a clave with non-return valves, allowing connection of propofol and remifentanil infusions using target-controlled infusion pumps. In our institution, the Schneider and Minto models are typically used, with effect-site targeting. Full routine intra-operative monitoring is commenced; invasive blood pressure measurement is very seldom used.
Surgical preparation
The patient is positioned supine with a pillow under their head in order to optimise the airway position for the supraglottic airway device and to make flexible bronchoscope insertion easier. If rigid bronchoscopy or suspension laryngoscopy is required, the pillow is removed.
The following instruments should always be available in case an alternative balloon delivery technique is required: (1) a correctly sized Trachealator non-occlusive balloon (DISA Medinotec, Johannesburg, South Africa) (available in sizes 6–18 mm) (Figure 2) – typically, a 16 mm or 18 mm balloon is required for adults; (2) a pressure inflator; (3) rigid tracheoscopes of sizes 6.5 mm, 7.5 mm and 8.5 mm (tracheoscopes are preferred over bronchoscopes to ensure a good seal during ventilation); and (4) suspension laryngoscopy equipment, in case dilatation via a supraglottic airway device is not possible.
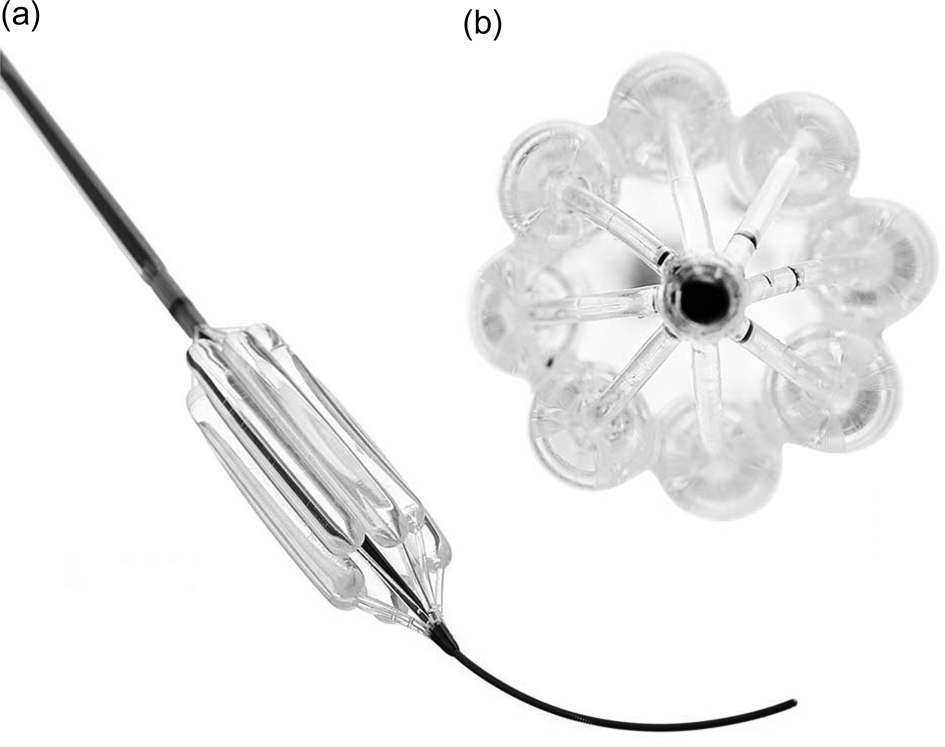
Fig. 2. Trachealator non-occlusive airway balloon. a = balloon advanced over guidewire; b = cross-sectional view of balloon showing subunit balloons creating inter-balloon space for ventilation
Procedure
After pre-oxygenation, target-controlled infusion and total intravenous anaesthesia is commenced, aiming for ablated airway reflexes and apnoea. No effort is made to maintain spontaneous ventilation. A supraglottic airway device is placed and seal-tested, the multiport airway adaptor is connected, and pressure-controlled ventilation is commenced and continued throughout the procedure (as demonstrated in a short video, available on The Journal of Laryngology & Otology website (Appendix 1) and in Figure 3).

Fig. 3. A still image from Appendix 1 showing the multidisciplinary team performing dilatation of tracheal stenosis with an inflated balloon in situ.
A well lubricated flexible bronchoscope or intubating video endoscope (ideally ‘paediatric’, with an external diameter of 3–4 mm) is passed via the multiport adapter through the supraglottic airway device and into the larynx. The vocal folds are sprayed with lignocaine lidocaine solution via the flexible bronchoscope before the latter is advanced through the vocal folds to identify the stenosis. The stenotic area (and distal airway) is sprayed with further lignocaine lidocaine to reduce stimulation during dilatation, and an assessment of the stenosis is made by the team.
The Cotton–Meyer grade, length of stenosis, distance from carina and vocal folds, and the nature of the stenosis (membranous or cartilaginous) are noted. Video and/or photographic documentation is obtained to compare with subsequent evaluations.
The Trachealator guidewire is passed through the bronchoscope until it is distal to the stenosis. The bronchoscope is removed with careful simultaneous advancement of the guidewire, leaving the guidewire in position. After the Trachealator balloon has been prepared and well lubricated, the balloon is passed over the guidewire through the multiport adaptor and then advanced into the supraglottic airway device. The flexible bronchoscope is then inserted through the second adaptor port, and follows the balloon as it is advanced through the supraglottic airway device into the trachea. The guidewire may occasionally need to be pulled back a few centimetres if the balloon does not advance easily. This implies that the guidewire has kinked in the distal airway, and pulling the guidewire back will straighten it and allow the balloon to slide easily through the stenosis. The flexible bronchoscope follows the balloon until the centre of the balloon is positioned in the middle of the stenosis. In very tight stenoses, this may cause brief obstruction of ventilation, which is relieved as soon as balloon inflation begins.
The non-occlusive balloon is steadily inflated to allow it to ‘grip’ the tracheal or bronchial wall. If ‘watermelon pipping’ occurs, making the balloon slip up or down out of the stenosis, the balloon is deflated, repositioned and re-inflated at a slower rate, with a firm grip applied to the balloon at the point of entry into the port. Once working pressure is obtained (usually 8 bar), a 3-minute countdown is begun. Ventilation through the supraglottic airway device should be easy, and can be increased if there has been a transient rise in end-tidal carbon dioxide. The balloon is deflated after 3 minutes, and an endoscopic assessment and photographic documentation are repeated. If needed, a second 3-minute dilatation is performed.
Target-controlled infusion and total intravenous anaesthesia are stopped as soon as the final dilatation is completed, and the patient is allowed to emerge from anaesthesia. Spontaneous ventilation can resume with the supraglottic airway device in situ. It is removed once the patient is awake and ready to be transferred to the recovery area.
Discussion
High-frequency jet ventilation is not currently available in South Africa. At our institution, the management of adult tracheobronchial stenosis has radically changed since we first began using the non-occlusive balloon in 2017.Reference Hofmeyr, McGuire, Park, Proxenos, Peer and Lehmann6 Use of the device has steadily increased, with more than 650 uses over the three-year period from 2019 to 2021, with; about a quarter of these occurring in our facility. During this time, we have not experienced any significant complications of the procedure. Long-term monitoring and outcome data for these patients are currently being collated in the Large Airway Interventional Registry (University of Cape Town Human Research Ethics Committee number: R018/2018).
Difficult anaesthesia and emergency tracheostomy are now often avoided by using the combination of a supraglottic airway device and an endoscopically guided balloon. A different technique using the balloon in suspension has been developed for paediatric patients.Reference Peer, Brooks and McGuire8 By performing a first dilatation under controlled conditions, a proper assessment of the stenosis can be made by a multidisciplinary team, and definitive treatment can be planned. In many cases, dilatation alone is sufficient, especially for membranous stenosis and in cases where early intervention is possible in the days following injury. A second bronchoscopy is usually scheduled six weeks after the first dilatation to evaluate the need for further dilatation or further surgical intervention, such as stenting or tracheal resection. By avoiding a tracheostomy, the tracheal area to be resected (in cases of firm cartilaginous stenosis) can be limited to the stenosis, shortening the length of tracheal resection. In the authors’ experience, if ‘watermelon pipping’ occurs despite correct positioning of the balloon and slow inflation, the chance of future surgical intervention (laser, reconstruction, resection or stenting) is high, regardless of which dilatation method is used (balloon, bronchoscope or bougie), as it indicates a firm cartilaginous stenosis.
Conclusion
Balloon dilatation often allows for adequate planning and elective surgery without the need for tracheostomy. The ease and safety of balloon dilatation through a supraglottic airway device has allowed otolaryngology trainees and junior anaesthesia staff to manage patients who present in airway extremis in a controlled environment, to secure the airway and allow for definitive planning by a multidisciplinary team.
Appendix 1. Supplementary video material
A short video of a typical case demonstrating the Cape Town technique is available online at The Journal of Laryngology & Otology website, at https://youtu.be/YtweHaimLME.
The supplementary material for this article can be found at https://doi.org/10.1017/S0022215122001426.
Competing interests
RH has no competing interests. DL was involved in the development of the device, is one of the patent inventors and has financial interests with the manufacturing company.