Introduction
Recent years have seen a growing interest in objectifying and measuring disease severity and treatment outcomes in the UK. Patient-reported outcome measures are increasingly being used to supplement objective clinical and/or biological measures of disease, as well as to measure the effectiveness of treatment. A recent review of patient-reported outcome measures in rhinology highlighted their influence and relevance, and concluded that ‘[patient-reported outcome measures] are likely to play an increasing role in measuring the success of medical treatments’.Reference Hopkins1 As clinicians are being increasingly called to account for their performance and quality of service rendered to patients, it appears very likely that patient-reported outcome measures will be used as part of this process.
Whilst various patient-reported outcome measures have been developed and implemented, these measures are usually used as research tools, and there has been resistance to their use in routine clinical practice. Barriers to such use include time pressures, logistical issues, and the conservative view that such outcome measures neither aid nor improve medical practice.
In the present study, we aimed to demonstrate the effective structuring and objectification of rhinology out-patient management by the use of a simple, practical, real-time, continuously updated, clinic-based electronic database containing symptom scores, clinical findings and treatment outcomes.
Materials and methods
We designed a concise database for use in our rhinology clinic. This database allowed the recording of: a structured, coded diagnosis; structured, coded examination findings; physiological measurements; and symptom scores. The results for each patient encounter were entered at the clinic into a Microsoft Access database, enabling integration of the recording of clinical and patient-reported outcome measurement. This database was loaded onto the secure hospital computer network, and was accessed by healthcare professionals via computer terminals in each consulting room. Using the open database connectivity facility of Microsoft Access, patient demographics could be easily transferred directly from the hospital electronic patient records database into the rhinology database (see Figure 1). Data recorded in the rhinology clinic included patient demographics, out-patient visit dates, diagnosis, Sino-Nasal Outcome Test 22 scores, pre- and post-decongestion nasal peak inspiratory flow (using Co-phenylcaine Forte spray (lignocaine hydrochloride 5 per cent, phenylephrine hydrochloride 0.5 per cent), two puffs in each nostril), and salivary cortisol levels.

Fig. 1 Screengrab showing the rhinology database ‘patient details’ entry fields.
The database contained a structured, gradable classification facilitating the recording of clinical diagnoses and examination findings. Available diagnostic groups included rhinitis, chronic rhinosinusitis (as defined by the Chronic Rhinosinusitis Task Force), chronic rhinosinusitis with polyps, anatomical anomaly, epistaxis and ‘other’ (see Figure 1).Reference Benninger, Ferguson, Hadley, Hamilos, Jacobs and Kennedy2 Listed options for endoscopic examination findings included no abnormality, structural abnormality, oedema, grade A to C nasal polyps, and pus (see Figure 2). As options appeared to the database user as ‘pop-up texts’, recording of data was simple and straightforward. The database also enabled the recording of the presence or absence of asthma as potentially significant co-morbidity.

Fig. 2 Screengrab showing the rhinology database ‘investigations’ entry fields, with sample data.
The Sino-Nasal Outcome Test 22 was chosen as the most suitable patient-reported outcome measure in terms of reliability, validity, responsiveness and ease of use.Reference Morley and Sharp3–Reference Hopkins, Gillett, Slack, Lund and Browne5 This outcome test addresses a broad range of health and health-related quality of life issues, including physical problems, functional limitations and emotional consequences. A recent pilot study of adults with no sinonasal disease concluded that a Sino-Nasal Outcome Test 22 score of 7 could be used as a guide for lack of sinonasal disease.Reference Gillett, Hopkins, Slack and Browne6
From January 2008, data were collected prospectively, using the above database, for all patients attending the Glasgow Royal Infirmary rhinology clinic. This clinic covered all aspects of rhinology in the adult population, and received referrals from throughout the Central Scotland catchment area.
During each rhinology out-patient visit, patients completed a Sino-Nasal Outcome Test 22 questionnaire in the waiting area prior to their consultation. Medical staff recorded each patient's clinical diagnoses, asthma status, Sino-Nasal Outcome Test 22 score, pre- and post-decongestion nasal peak inspiratory flow, and endoscopic examination findings, using a designated recording sheet. Clinical nursing staff then assisted medical staff in entering data into the rhinology database, at the time of consultation.
Nasal peak inspiratory flow was used as a simple and practical way of measuring nasal obstruction. This parameter has been found to correlate with symptom severity scores.Reference Holmstrom, Scadding, Lund and Darby7 It is also comparable to other objective measurements of the nasal airway such as acoustic rhinometry and rhinomanometry, which are mainly employed as research tools and are not routinely utilised in the clinic setting for practical reasons.
Nasal polyps were graded as A, B or C, using the system described by Lund and MacKay in 1993.Reference Lund and MacKay8 Grade A polyps comprise those confined to the middle meatus. Grade B polyps extend beyond the middle meatus but do not completely obstruct the nasal cavity. Grade C polyps completely obstruct the nasal cavity. If the polyp size was asymmetrical, it was graded according to the worse side.
In some patients, topical intranasal glucocorticoids may cause occult adrenal insufficiency, and assessment of adrenal function is recommended in these patients.Reference Patel, Wallace, Hinnie and McGarry9 We used salivary cortisol measurement for this purpose, as it has been found to be a useful, non-invasive and economical test for monitoring patients using intranasal corticosteroids. The positive predictive value of this test for iatrogenic adrenal suppression is 100 per cent.Reference Patel, Wallace, Hinnie and McGarry9
Results
Data from 770 consecutive patients (390 males and 380 females) were entered into the rhinology database between January 2008 and April 2009. The mean patient age was 45 years (range, 13–89 years). Patient diagnosis groupings were as follows: rhinitis (20.4 per cent), chronic rhinosinusitis (12.2 per cent), chronic rhinosinusitis with polyps (24.7 per cent), anatomical anomaly (22.7 per cent), epistaxis (3.4 per cent) and other (18.4 per cent). Some patients had more than one diagnosis, e.g. rhinitis and anatomical anomaly (n = 19). Asthma was noted in 20.6 per cent of all patients, 38.4 per cent of those with chronic rhinosinusitis plus polyps, 14.9 per cent of those with chronic rhinosinusitis and 21.7 per cent of those with rhinitis. The proportion of asthmatic patients in the chronic rhinosinusitis with polyps group was significantly higher compared with both the chronic rhinosinusitis and the rhinitis groups (p < 0.001 for both, Mann–Whitney U test). There was no statistically significant difference in the proportion of asthmatic patients in the chronic rhinosinusitis versus rhinitis groups.
Sino-Nasal Outcome Test 22 scores by gender and diagnostic category
The overall mean Sino-Nasal Outcome Test 22 score was 41.4 for males and 45.0 for females. Table I shows mean Sino-Nasal Outcome Test 22 scores by gender and diagnostic category. Female patients' mean scores were higher than male patients' scores for all diagnostic groups apart from chronic rhinosinusitis with polyps; however, these differences were not statistically significant.
Table I Patients' SNOT-22 scores by gender and diagnostic category

Data represent means (standard deviations). SNOT-22 = Sino-Nasal Outcome Test 22; CRS = chronic rhinosinusitis; P = polyps; anat anom = anatomical anomaly
We do not report Sino-Nasal Outcome Test 22 scores for patients in the ‘other’ category, as this was a very heterogeneous group containing patients with nasopharyngeal tumour, sarcoidosis, Wegener's granulomatosis, cerebrospinal fluid leakage etc. As far as we were aware, the use of Sino-Nasal Outcome Test 22 scores has only been validated in the chronic rhinosinusitis population. We did however use the Sino-Nasal Outcome Test 22 in patients with chronic rhinosinusitis plus polyps, chronic rhinosinusitis and rhinitis, as we felt these cases constituted a spectrum of the same or similar disease processes.
Effect of medical therapy by diagnosis
The mean first visit Sino-Nasal Outcome Test 22 scores for patients with rhinitis, chronic rhinosinusitis and chronic rhinosinusitis plus polyps were 47, 45.7 and 46.8, respectively (see Table II and Figure 3). These patients were all treated medically, with no surgical intervention. The interval between these patients' visits was eight to 12 weeks. Comparing first and second visits, the greatest improvement in mean Sino-Nasal Outcome Test 22 score occurred in the chronic rhinosinusitis with polyps group (−11.3), followed by the rhinitis group (−6.1) and the chronic rhinosinusitis group (−5.4). This score improvement was statistically significant in the chronic rhinosinusitis plus polyps group (p < 0.0001, t-test) and the rhinitis group (p = 0.029, t-test), but not in the chronic rhinosinusitis group.

Fig. 3 Patients' mean Sino-Nasal Outcome Test 22 (SNOT-22) scores for each clinic visit, by diagnostic category. CRS = chronic rhinosinusitis; P = polyps
Table II Patients' mean SNOT-22 scores by diagnostic category and out-patient visit

SNOT-22 = Sino-Nasal Outcome Test 22; pts = patients; SD = standard deviation; CRS = chronic rhinosinusitis; P = polyps
Figure 3 shows the sustained improvement observed in the chronic rhinosinusitis plus polyps patients over subsequent clinic visits, in contrast to the rhinitis and chronic rhinosinusitis groups. In the chronic rhinosinusitis group, mean Sino-Nasal Outcome Test 22 scores appeared to plateau after the second visit.
Effect of asthma on treatment of chronic rhinosinusitis plus polyps patients
The chronic rhinosinusitis plus polyps group comprised 113 males and 76 females. In this group, 38.4 per cent of patients were asthmatic. Both the asthmatic and non-asthmatic patients of this group showed a statistically significant decrease in their Sino-Nasal Outcome Test 22 scores over time. In this group, at the fourth visit, the small remaining cohort of asthmatic patients had a higher mean Sino-Nasal Outcome Test 22 score than non-asthmatic patients, but this difference was not statistically significant (see Figure 4).

Fig. 4 Mean Sino-Nasal Outcome Test 22 (SNOT-22) scores for patients with chronic rhinosinusitis plus polyps (CRS + P) for each clinic visit, by asthma (A) status.
Effect of polyp grade on Sino-Nasal Outcome Test 22 score
A correlation was found between polyp grading and Sino-Nasal Outcome Test 22 scores. The mean scores for patients with grade A, B and C polyps were 38.2, 42.5 and 50.6, respectively; the differences between these scores were statistically significant (one-way analysis of variance, p = 0.0045). There was also a statistically significant linear trend. It has not previously been demonstrated that Sino-Nasal Outcome Test 22 scores are able to differentiate between patients with different polyp gradings. In our patients, we found that a worse polyp grading correlated with a higher Sino-Nasal Outcome Test 22 score. There was a difference of approximately 12 points between the Sino-Nasal Outcome Test 22 scores of patients with grade A and grade C polyps. Interestingly, this is approximately the same mean difference in Sino-Nasal Outcome Test 22 scores achieved following one clinic visit in the chronic rhinosinusitis with polyps patients.
Effect of surgery on Sino-Nasal Outcome Test 22
Patients who had undergone surgical procedures showed a difference in their pre-operative versus post-operative Sino-Nasal Outcome Test 22 scores (see Table III). The average time interval between the post-operative Sino-Nasal Outcome Test 22 completion date and the operation date was 63 days. Unfortunately, we can report data for only a relatively small number of patients, as those with incomplete pre- or post-operative Sino-Nasal Outcome Test 22 scores were excluded. These small numbers were due to inconsistent data input at the early stages of clinical implementation of the electronic database.
Table III Patients' mean SNOT-22 scores by diagnostic category and surgical status
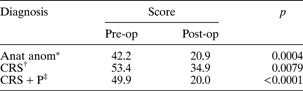
*n = 20; †n = 14; ‡n = 24. SNOT-22 = Sino-Nasal Outcome Test; pre-op = pre-operative; post-op = post-operative; anat anom = anatomical anomaly; CRS = chronic rhinosinusitis; P = polyps
Those patients who underwent surgical treatment of their rhinological conditions reported greater pre-operative disability, reflected by higher Sino-Nasal Outcome Test 22 scores, compared with those treated only medically. This indicated that these patients (i.e. those with chronic rhinosinusitis and chronic rhinosinusitis plus polyps) were more symptomatic and had failed to improve with medical therapy. Post-operatively, these patients showed a marked improvement in their Sino-Nasal Outcome Test 22 scores (see Table III).
Discussion
This study demonstrates the practical challenges of adopting a structured approach to rhinology out-patient consultations, and the value of a structured clinical rhinology database. The accessibility and simplicity of the database enabled us to integrate patient's clinical findings with their self-reported outcome measures.
A similar database – the British Rhinological Society Rhinology Minimum Electronic Database – has only very recently become accessible to ENT-UK members, and enables the prospective collection of rhinology patient outcomes data.10, 11 This electronic database has been approved for use by the UK General Medical Council and the Department of Health Audit Advisory Group, for appraisal and re-licensing as well as to provide outcome measures for contracting.11 Our database was an ‘in house’ attempt to address this same need, developed prior to the above database.
In view of the increasing requirement to record outcome measures, it is essential to have in place an effective tool with which to capture such data. As it is neither feasible nor desirable for each unit to develop its own database, we support the use of the British Rhinology Society database, as this is now readily available and allows uniformity of data collection as well as comparability of data between units. Access to the demonstration version of this database is available via the British Rhinology Society online rhinology database guidance notes.11
As with our own rhinology database, data entry into the British Rhinology Society database is straightforward. An added feature of our database was its linkage to the hospital patient database, enabling simple transferral of new patients' demographic data into the rhinology database. As for the time burden of data entry, it took no more than 1.5 minutes to enter information from each patient's recording sheets onto our database.
At the time of writing, information was still being collected at each clinic session, enabling us to gather long-term data and to continue to assess the effectiveness of our medical and surgical management. The collected dataset enables us to characterise our rhinology clinic patient population; for example, one-quarter of attending patients have chronic rhinosinusitis with polyps, and rhinitis patients and those with an anatomical anomaly (e.g. septal deviation, adhesion or perforated septum) together constitute one-fifth of our clinic population.
Analysis of Sino-Nasal Outcome Test 22 scores showed that the highest level of symptoms was reported by patients with rhinitis, followed by those with chronic rhinosinusitis plus polyps and those with chronic rhinosinusitis; however, this difference was not statistically significant. It was surprising that rhinitis patients reported higher scores, as one would have regarded patients with chronic rhinosinusitis plus polyps as being at the more severe end of the disease profile. Formal sub-score analysis was not performed in the current study, but preliminary data perusal appeared to indicate that rhinitic patients scored worse in the sleep and psychological domains of the Sino-Nasal Outcome Test 22 questionnaire. Further sub-score analysis is being undertaken to assess the sub-scale scores of patients in the various diagnostic groupings. This analysis may provide further insights on how to improve patients' symptoms and quality of life.
In the rhinitis group, we noted that treatment non-responders had similar Sino-Nasal Outcome Test 22 scores at their second, third and fourth clinic visits, compared with their first visit. In contrast, those who did respond to initial topical steroid therapy showed corresponding improvements in their Sino-Nasal Outcome Test 22 scores at the second visit. The majority of rhinitis patients (78.3 per cent) were discharged after their second clinic visit.
• Measurements of disease severity and medical intervention effectiveness are not routinely recorded in the rhinology clinic
• A prospectively recorded, computerised database is a simple, effective, practical tool for integrating the recording of clinical and patient-reported outcome measures
• The reported database enables on-going assessment of treatment effectiveness within the rhinology out-patient clinic
Our preliminary Sino-Nasal Outcome Test 22 data showed that patients with chronic rhinosinusitis plus polyps gained most benefit from medical treatment. This may be due to the use of higher potency steroid medications, such as betamethasone drops rather than sprays. Due to the risk that nasal drops may have greater systemic effects than nasal sprays, patients using nasal steroid drops (e.g. betamethasone sodium phosphate 0.1 per cent) were included in our salivary cortisol monitoring programme, to detect iatrogenic adrenal suppression.Reference Patel, Wallace, Hinnie and McGarry9
Our patients with chronic rhinosinusitis with polyps plus asthma appeared to respond well to treatment. In this rhinological diagnostic group, there was no significant difference in the mean Sino-Nasal Outcome Test 22 scores of asthmatic versus non-asthmatic patients. This contrasts with previous evidence suggesting that coexistent asthma impairs treatment response.Reference Larsen12, Reference Seybt, McMains and Kountakis13
The Sino-Nasal Outcome Test 22 scores of patients with chronic rhinosinusitis and chronic rhinosinusitis plus polyps appeared to plateau after the second clinic visit. Long-term data are being collected but have not yet been analysed; however, it would seem that, in such patients, the mean Sino-Nasal Outcome Test 22 score varies between 30 and 40. Once long-term data are available for further analysis, it may be possible for these patients to be followed up by their general practitioners, and to be reviewed in the rhinology clinic only if their Sino-Nasal Outcome Test 22 scores increase beyond a defined ‘trigger’ value.
Conclusion
We have demonstrated that the described rhinology database provides a simple, effective and practical tool for integrating the recording of clinical outcome parameters and patient-reported outcome measures. This database has enabled us to analyse the characteristics of our rhinology out-patient population, and has provided information on how better to improve and target our rhinology services. More importantly, the database has enabled us to measure and demonstrate the effectiveness of patient treatments, in terms of improved symptoms and quality of life.
Acknowledgement
The authors would like to thank Susanne Blythe and all the ENT clinic nursing staff for their help in this research project.









