Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Joubert, P. H.
Pretorius, S. J.
and
Kruger, F. J.
1991.
Further studies on the susceptibility ofBulinus africanusto infection withSchistosoma haematobium.
Annals of Tropical Medicine & Parasitology,
Vol. 85,
Issue. 2,
p.
253.
Khallaayoune, K.
and
Laamrani, H.
1992.
Seasonal patterns in the transmission of Schistosoma haematobium in Attaouia, Morocco.
Journal of Helminthology,
Vol. 66,
Issue. 2,
p.
89.
Kabatereine, Narcis B.
Fleming, Fiona M.
Nyandindi, Ursuline
Mwanza, James C.L.
and
Blair, Lynsey
2006.
The control of schistosomiasis and soil-transmitted helminths in East Africa.
Trends in Parasitology,
Vol. 22,
Issue. 7,
p.
332.
Agnew-Blais, J.
Carnevale, J.
Gropper, A.
Shilika, E.
Bail, R.
and
Ngoma, M.
2010.
Schistosomiasis Haematobium Prevalence and Risk Factors in a School-age Population of Peri-urban Lusaka, Zambia.
Journal of Tropical Pediatrics,
Vol. 56,
Issue. 4,
p.
247.
Mazigo, Humphrey D
Nuwaha, Fred
Kinung’hi, Safari M
Morona, Domenica
de Moira, Angela Pinot
Wilson, Shona
Heukelbach, Jorg
and
Dunne, David W
2012.
Epidemiology and control of human schistosomiasis in Tanzania.
Parasites & Vectors,
Vol. 5,
Issue. 1,
Virginia Fernández, María
Inés Hamann, Mónika
and
Ostrowski-de Nóñez, Margarita
2013.
Larval trematodes of Biomphalaria straminea (Mollusca: Planorbidae) in a ricefield in Corrientes Province, Argentina.
Revista Mexicana de Biodiversidad,
Vol. 84,
Issue. 3,
p.
756.
Hastings, Julie
2016.
RUMOURS, RIOTS AND THE REJECTION OF MASS DRUG ADMINISTRATION FOR THE TREATMENT OF SCHISTOSOMIASIS IN MOROGORO, TANZANIA.
Journal of Biosocial Science,
Vol. 48,
Issue. S1,
p.
S16.
Angelo, Teckla
Buza, Joram
Kinung’hi, Safari Methusela
Kariuki, Henry Curtis
Mwanga, Joseph Rogathe
Munisi, David Zadock
and
Wilson, Shona
2018.
Geographical and behavioral risks associated with Schistosoma haematobium infection in an area of complex transmission.
Parasites & Vectors,
Vol. 11,
Issue. 1,
Ndassi, Vicky Daonyle
Anchang-Kimbi, Judith Kuoh
Sumbele, Irene Ule Ngole
Wepnje, Godlove Bunda
and
Kimbi, Helen Kuokuo
2019.
Prevalence and Risk Factors Associated withS. haematobiumEgg Excretion during the Dry Season, Six Months following Mass Distribution of Praziquantel (PZQ) in 2017 in the Bafia Health Area, South West Region Cameroon: A Cross-Sectional Study.
Journal of Parasitology Research,
Vol. 2019,
Issue. ,
p.
1.
Hailegebriel, Tamirat
Nibret, Endalkachew
and
Munshea, Abaineh
2020.
Prevalence of Schistosoma mansoni and S. haematobium in Snail Intermediate Hosts in Africa: A Systematic Review and Meta-analysis.
Journal of Tropical Medicine,
Vol. 2020,
Issue. ,
p.
1.
Fuss, Antje
Mazigo, Humphrey Deogratias
and
Mueller, Andreas
2020.
Malacological survey to identify transmission sites for intestinal schistosomiasis on Ijinga Island, Mwanza, north-western Tanzania.
Acta Tropica,
Vol. 203,
Issue. ,
p.
105289.
Mazigo, Humphrey D.
Mwingira, Upendo J.
Zinga, Maria M.
Uisso, Cecilia
Kazyoba, Paul E.
Kinung’hi, Safari M.
Mutapi, Francesca
and
Shiff, Clive
2022.
Urogenital schistosomiasis among pre-school and school aged children in four districts of north western Tanzania after 15 years of mass drug administration: Geographical prevalence, risk factors and performance of haematuria reagent strips.
PLOS Neglected Tropical Diseases,
Vol. 16,
Issue. 10,
p.
e0010834.
Mazigo, Humphrey D.
Zinga, Maria M.
Kepha, Stella
Yard, Elodie
McRee-Mckee, Kevin
Kabona, George
Ngoma, Deogratias D.
and
Nshala, Andreas
2022.
Precision and geographical prevalence mapping of schistosomiasis and soil-transmitted helminthiasis among school-aged children in selected districts of north-western Tanzania.
Parasites & Vectors,
Vol. 15,
Issue. 1,
Lee, Jungim
Cha, Seungman
Cho, Yoonho
Musiba, Anold
Marwa, Boniphace
and
Mazigo, Humphrey
2023.
Prevalence of Schistosomiasis and Soil-Transmitted Helminthiasis and Their Risk Factors: A Cross-Sectional Study in Itilima District, North-Western Tanzania.
Life,
Vol. 13,
Issue. 12,
p.
2333.
Mbwanji, Gladys
Mazigo, Humphrey D.
Maganga, Jane K.
Downs, Jennifer A.
and
Ekpo, Uwem Friday
2024.
Female genital schistosomiasis is a neglected public health problem in Tanzania: Evidence from a scoping review.
PLOS Neglected Tropical Diseases,
Vol. 18,
Issue. 3,
p.
e0011954.
Mazigo, Humphrey D.
Kayange, Neema
Ambrose, Emmanuela E.
Zinga, Maria M.
Mugassa, Stella
Ruganuza, Deodatus
Mwingira, Upendo J.
Uisso, Cecilia
and
Mutapi, Francesca
2024.
Efficacy of praziquantel drug against Schistosoma haematobium and performance of urine reagent strips among pre-and-school aged children during the high transmission season in North-Western Tanzania.
Acta Tropica,
Vol. 256,
Issue. ,
p.
107232.
Justine, Nyanda C.
Mazigo, Humphrey D.
Fuss, Antje
Webster, Bonnie L.
Konje, Eveline T.
Brehm, Klaus
and
Mueller, Andreas
2025.
Seasonal distribution and cercarial shedding of Bulinus spp. snails: Implications for urogenital schistosomiasis control in the Simiyu Region, northwestern Tanzania.
Current Research in Parasitology & Vector-Borne Diseases,
Vol. 7,
Issue. ,
p.
100248.
Mazigo, Humphrey
Lee, Jungim
Cho, Yoonho
Cha, Seungman
and
Jin, Yan
2025.
Changes in schistosomiasis prevalence after 2 years of an integrated intervention in the Itilima district of Tanzania.
Parasites, Hosts and Diseases,
Vol. 63,
Issue. 1,
p.
75.
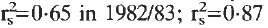 in 1983/84) suggesting that the maximum transmission period for urinary schistosomiasis in the area occurs during the short dry period, sometime in February/March so that most of the infections in the community would be detected in April/May.
in 1983/84) suggesting that the maximum transmission period for urinary schistosomiasis in the area occurs during the short dry period, sometime in February/March so that most of the infections in the community would be detected in April/May.