Introduction
Fish parasitoses act as a potential factor restraining the growth of the fish productivity. Some helminths of fish may also be zoonoses and, therefore, represent a public health problem. The Caspian Sea has a north–south positive gradient of water salinity, from freshwater salinity in the north basin, which has most of the freshwater inflow, to an almost homogeneous 12.5–13.5 parts per thousand surface water salinity in the middle and south basins (Leroy et al., Reference Leroy, Marret, Gibert, Chalié, Reyss and Arpe2007). The north Caspian Sea and associated drainage basin is the most important fishery of Kazakhstan, with about 0.3 million tons of fish caught annually. In total, 80 species or subspecies of fish are found in the northern part of the Caspian Sea (Naseka & Bogutskaya, Reference Naseka and Bogutskaya2009). These include freshwater species found close to the coast and rivers of the drainage basis, as well as marine species found in areas of higher salinity. Of these, 20 species are being developed by industry. Over a ten-year period, from 2010 to 2019, between 4.5 and 14.5 thousand tons of fish were caught per annum by Kazakhstani fishermen from the Caspian Sea. The largest catches were for bream (25%), carp (18.8), roach (15.9%) and pike perch (9.8%). For 2019, in the Zhaiyk River commercial fish catches included 1970 tons of bream, 670 tons of roach, 340 tons of pike perch, 250 tons of asp, 180 tons of Prussian carp and 138 tons of carp (Assylbekova et al, 2020). Therefore, it is important to understand the diseases of fish from this region. Such studies may also contribute to ameliorating the public health risk of some helminths as some species such as the asp and pike perch may be heavily infected with Anisakis spp. (Abdybekova et al., Reference Abdybekova, Abdibayeva, Popov, Zhaksylykova, Barbol, Bozhbanov and Torgerson2020).
Previous studies on the parasites of fish of commercial importance include a number of studies from the southern sectors of the Caspian, mainly from Iran (Khara et al., Reference Khara, Sattari, Nezami, Mirhasheminasab, Mousavi and Ahmadnezhad2011; Mazandarani et al., Reference Mazandarani, Hajimoradloo and Niazi2016). Studies of fish parasites in the Soviet sector of the Caspian Sea were first carried out in 1931–1932 by Dogel & Bykhovsky (Reference Dogel and Bykhovsky1939) and, more recently, by Tokpan & Rakhimov (Reference Tokpan and Rakhimov2010).
There is less information on the distribution of parasites within host species of fish and how these may provide clues to the dynamics of parasite infection. The simplest of these is the Poisson model. This would be consistent with the fish becoming infected by individual parasites at random. Such a distribution of parasites in their hosts are rarely seen, although they have been described with adult Taenia spp. cestodes in dogs (Lahmar et al., Reference Lahmar, Kilani and Torgerson2001). Generally, parasite infection of fish is overdispersed, with a small proportion of the population being heavily infected and the remainder having few or no parasites (see, e.g., Burrough, Reference Burrough1978). This is seen in common parasites of terrestrial vertebrates, which also tend to have an overdispersed parasite distribution (Grenfell et al., Reference Grenfell, Wilson, Isham, Boyd and Dietz1995). Various probability models can be used to describe this overdispersed distribution of parasites in hosts. The zero-inflated Poisson distribution is an overdispersed distribution where there is an excess of zeros compared to a Poisson distribution. Here, this can be hypothesized to be due to two processes: a proportion of the host population is not exposed to the infectious stage of the parasite, whilst the rest of the population is exposed at random. Again, there are limited examples of parasites of terrestrial vertebrates that are consistent with a zero-inflated Poisson distribution (e.g. Abdybekova & Torgerson, Reference Abdybekova and Torgerson2012). A negative binomial distribution is consistent with a clumped infection process, although other processes such as variations in resistance to infection between individual hosts may also play a role. A negative binomial model is the most commonly used model and is the extension of a Poisson model for integer counts and can be used to model distributions where the variance of the mean is in excess of the mean abundance. There are numerous examples where parasite distributions have been modelled as a negative binomial distribution both within fish species (e.g. Burrough, Reference Burrough1978; Balling & Pfeiffer, Reference Balling and Pfeiffer1997) and with terrestrial vertebrates (e.g. Grenfell et al., Reference Grenfell, Wilson, Isham, Boyd and Dietz1995; Wilson et al., Reference Wilson, Grenfell and Shaw1996). The zero-inflated negative binomial distribution is when there is an excess of zeros compared to the negative binomial distribution, and, again, this may occur if the host population is partitioned into one group that is not exposed and another that is exposed to a clumped infection process, or there is a variation in resistance amongst exposed hosts. There are a limited number of studies in fish using the zero-inflated negative binomial, but do include attached sea lice on sea trout (Vollset et al., Reference Vollset, Qviller, Skår, Barlaup and Dohoo2018). For terrestrial vertebrates, such models have been used, for example, to model the distribution of helminths of wild carnivores (Ziadinov et al., Reference Ziadinov, Deplazes, Mathis, Mutunova, Abdykerimov, Nurgaziev and Torgerson2010) and for parasitic faecal egg counts in agricultural animals (Denwood et al., Reference Denwood, Stear, Matthews, Reid, Toft and Innocent2008). A better understanding of such dynamics leads to a deeper understanding of transmission biology.
A previous, more limited study failed to find any association between the condition of the fish and the intensity of infection of individual fish (Abdybekova et al., Reference Abdybekova, Abdibayeva, Popov, Zhaksylykova, Barbol, Bozhbanov and Torgerson2020). Using additional data collected over a further two years, we were able to explore these effects more fully. We were also able to analyse any association with the age of fish, which might give further clues on the infection dynamics and relative resistance to infection. Furthermore, we aimed to identify potential pathogenic effects by analysing the association of Fulton's condition index with the intensity of infection with parasites identified. In these analyses, it was essential to utilize the best generalized linear model (GLM), taking into account the aggregated or zero-inflated distribution to gain inferences with regard to any of these associations.
Materials and methods
In total, 1597 individuals of ten fish species were investigated from the Kazakhstan sector of the Caspian Sea from 2018 to 2020. All fish were collected in late spring to early summer. These included 450 fish collected in 2018 reported in a preliminary study (Abdybekova et al., Reference Abdybekova, Abdibayeva, Popov, Zhaksylykova, Barbol, Bozhbanov and Torgerson2020). The species composition of the fish was determined on the basis of taxonomic descriptions according to Berg (Reference Berg1949), Kazancheyev (Reference Kazancheyev1981) and Reshetnikova (Reference Reshetnikova2002). A complete biological analysis of the fish was carried out with the determination of the length, mass, sex and maturity stages of the gonads (Pravdin, Reference Pravdin1966). The ages of the fish were determined by rings on the scales or otoliths, or by cuts of the marginal rays of the pectoral fins (Chugunova, Reference Chugunova1959; Konoplev, Reference Konoplev1975). The body length of all fish was measured from the top of the snout to the end of the scaly cover and to the end of the caudal fin. Fish were weighed on an electronic scale with an accuracy of 1 g. For small fish, this was with an accuracy of 0.1 g. Fulton's condition index (F) was calculated for each fish as:
where W = the weight in grams and L is the length in cm (Nash et al., Reference Nash, Valencia and Geffen2006).
In the field, a complete parasitological dissection of fish was carried out according to the standard classical method (Skriabin, Reference Skriabin1928; Dogel, Reference Dogel1933; Bykhovskaya-Pavlovlskaya, Reference Bykhovskaya-Pavlovlskaya1969). The results of the autopsies of the fish were recorded. These included the fish species, the place of investigation, sex, age, weight of the fish and the number, species and localization of detected parasites. Fish muscles and all internal organs were examined under a KRUSSMSZ5000 stereomicroscope (Krüss Optronic, Hamburg, Germany) with a range of 7–45×. Parasites were fixed in various fixatives: monogeneans, trematodes, cestodes and parasitic crustaceans in 700 alcohol, and nematodes in Barbagallo fluid. For species identification, nematodes were placed in a solution of glycerol with water (1:1) in order to clear them and then view the internal structure of helminths. This, therefore, enabled taxonomic identification based on the morphological features of the parasites.
Four different models of the probability distribution were analysed. The Poisson model assumes fish are randomly infected, whilst the negative binomial model models overdispersion and is consistent with fish being infected with a clumped infection pressure. The zero-inflated model also models overdispersion, but is consistent with fish belonging to two classes. With the zero-inflated Poisson model, fish are not exposed to infection with parasites and, so, the zero-inflated component consists of having no parasites. The other class are fish which are exposed at random and, consequently, the non-zero inflated partition will have a Poisson distribution of parasites. Finally, the negative zero-inflated binomial distribution has a zero-inflated partition, where fish are not infected, whilst the fish in the other partition have an overdispersed distribution of parasites consistent with a clumped infection process or variability between susceptibility of infection in different individual fish. The data were fit to these four different models using the pscl library in R (Zeileis et al., Reference Zeileis, Kleiber and Jackman2008). To investigate any effects of parasitism on the fish, a GLM was used to analyse the association of the intensity of infection of each individual fish using the appropriate probability distribution. For each parasite, a multivariable GLM investigated the association of parasite abundance with the Fulton index, age, gender, year and location of where the fish was caught. Likewise, for the zero-inflated models, the magnitude of the zero inflation was examined for an association with each of these variables. A backward selection method was used with all variables included in the initial model, with each non-significant variable with a P > 0.15 being removed sequentially, and only significant variables remaining in the final model. If two or more independent variables remained in the model, it can be interpreted that all these variables had an association with the dependent variable. The regression model with the lowest Akaike information criterion was used to select the most parsimonious model. The relative difference in the odds of the zero-inflated proportion of the infection of fish with parasites according to the risk factors is reported as an odds ratio. The relative difference of abundances between fish according to the risk factors in the non-zero inflated proportion is reported as the incidence rate ratio (IRR). All analyses were undertaken in R (R Core Team, 2019).
Results
The results are summarized in table 1. The prevalences of infection ranged from 1% for the common bream with the parasite Philometra abdominalis to 64% for infection of carp with Anisakis schupakovi. Abundances were also highly variable, ranging from 0.12 parasites per fish (i.e. about one parasite for every eight fish) for Khawia sinensis infection of the common bream to 66 per fish for A. schupakovi in the carp. The most likely frequency distribution of each parasite according to host species is given in table 1.
Table 1. Probability distributions and factors associated with parasite abundance from ten species of fish.
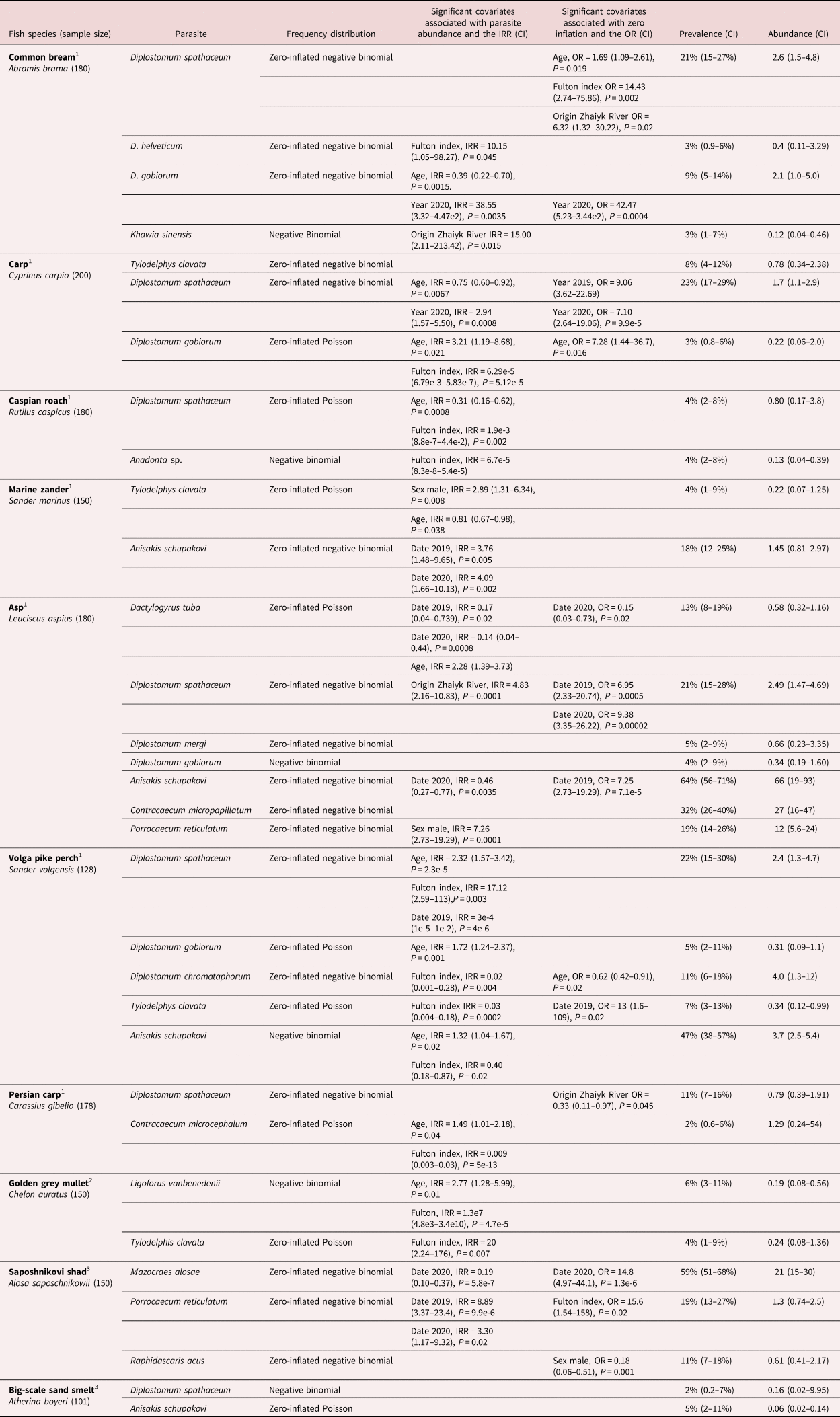
1 Seven of these are found in the low-saline environment of the north Caspian and Zhaiyk rivers.
2 One marine species has been introduced (Naseka & Bogutskaya, Reference Naseka and Bogutskaya2009).
3 Two marine species are endemic to the Caspian.
IRR, incidence rate ratio; CI, confidence interval; OR, odds ratio.
An increase in parasite abundance was associated with an increase in the Fulton index (IRR > 1) for Diplostomum helveticum and P. abdominalis infection of the common bream; Diplostomum spathaceum infection of the Volga pike perch; and Ligoforus vanbenedenii and Tylodelphis clavata infection of the golden grey mullet. In contrast, increasing parasite abundance was associated with a decrease in the Fulton index for Diplostomum gobiorum infection in carp; D. spathaceum infection in the Caspian roach; and Contracecum microcephalum infection in the Prussian carp.
Variations in parasite abundance with age were seen in a number of the fish species. The abundance of D. gobiorum in the common bream decreased with increasing age; D. spathaceum in carp and the Caspian roach; and T. clavata in the marine pike perch. In contrast, increases in abundance associated with increasing age were seen with D. gobiorum in carp; Dactylogyrus tuba in the asp; D. spathaceum, D. gobiorum and A. schupakovi in the Volga pike perch; C. microcephalum in the Prussian carp; and L. vanbenedenii in the golden grey mullet.
Variations in the abundance according to sex of the fish were seen with T. clavata infection of the marine zander and with Porrocaecum reticulatum infection in the asp. In both instances, the males were more heavily infected than the females.
Variations in the abundance of parasites with the year in which the fish were recovered were seen on a number of occasions. The year 2020 had the most intensely infected bream with D. gobiorum; the most intensely infected carp with D. spathaceum; and the most intensely infected marine zander with A. schupakovi. The year 2018 had the most intensely infected asp with D. tuba and A. schupakovi; the most intensely infected Volga pike perch with D. spathaceum; and the most intensely infected Saposhnikovi shad with Мazocraes alosae. The year 2019 had the most intensely infected asp with D. spathaceum and the most intensely infected Saposhnikovi shad with P. reticulatum.
Higher abundances of K. sinensis in bream and D. spathaceum in asp were found in fish from the Zhaiyk River compared to the northern Caspian.
Discussion
In the present study, we have examined four models to describe the frequency distribution of various parasites in a sample of a number of different species of fish. We did not observe any parasite species whose distribution was consistent with a Poisson distribution and, thus, a completely random distribution. We observed a zero-inflated Poisson distribution in several fish species infected with parasite (see table 1). The zero-inflated Poisson distribution suggests that the infection is not clumped and would be consistent with intermediate hosts being infected with single larvae, given that the host is exposed to possible infection.
A negative binomial distribution is consistent with a clumped infection process and all fish potentially being exposed. This type of distribution was seen in a number of fish species infected by various parasites. Finally, the zero-inflated negative binomial distribution is consistent with a clumped infection process, but only a proportion of the fish population being exposed. This distribution was the most commonly seen distribution.
Diplostomum spp. are trematode parasites and fish are infected by cercariae which are released into the water by snail intermediate hosts. Infected snails can release large numbers of cercariae into the water – for D. spathaceum, a mean number of 5199 cercariae per snail per day was reported by Vyhlídalová & Soldánová (Reference Vyhlídalová and Soldánová2020). For a Poisson process to result in infection of fish, it can be hypothesized that the cercariae are likely to be dispersed in the water and encounter the fish at random. Alternatively, there could be parasite-induced mortality, which removes heavily infected fish from the population; in which case, the infection process is not random, but the resulting distribution in the fish appears to be random. For a negative binomial process, it can be hypothesized that the cercariae are not randomly dispersed in water but may be at a greater density in certain habitats with heavily infected fish more likely to frequent these habitats. Since D. spathaceum cercariae are not actively host-seeking and are often patchily distributed, behavioural avoidance of these sources of infestation is the prime defence mechanism displayed by fish hosts (Karvonen et al., Reference Karvonen, Seppälä and Valtonen2004a). Thus, the zero-inflated distributions of some of these parasites are consistent with the avoidance of these patches of cercariae by some of these wild-caught fish. Alternatively, the overdispersed distribution in fish may be related to variations in susceptibility of the fish to the parasite, resulting in a proportion of the fish population having a lower or zero burden of the parasite despite exposure.
With ocular trematodes, parasite metacercariae induce cataracts due to mechanical destruction of the lens and metabolic products excreted by the parasites, thus reducing the host's vision (Karvonen et al., Reference Karvonen, Seppälä and Valtonen2004b; Seppälä et al., Reference Seppälä, Karvonen and Tellervo Valtonen2004). Consequently, an induced increase in susceptibility to predation with fish having heavier infections of Diplostomum species is due to reduced predator avoidance behaviour (e.g. Brassard et al., Reference Brassard, Rau and Curtis1982a; Flink et al., Reference Flink, Behrens and Svensson2017). In addition, Diplostomum have been reported as having a direct pathogenic effect, with an exponential increase in mortality as infection intensity increases (Brassard et al., Reference Brassard, Rau and Curtis1982b). Likewise, infection with T. clavata has been suggested to negatively affect the feeding behaviour of perch, which is hypothesized to increase the risk of predation (Vivas Muñoz et al., Reference Vivas Muñoz, Bierbach and Knopf2019). Rainbow trout, Oncorhynchus mykiss, infected with D. spathaceum engage in behaviours that put them at greater risk of predation. For example, those harbouring the parasite appear to be more vulnerable to a simulated predator attack (Seppälä et al., Reference Seppälä, Karvonen and Tellervo Valtonen2004, Reference Seppälä, Karvonen and Valtonen2008). Additionally, when provided with the choice of a dark or light background, infected trout spent more time over the light area, which makes them visually more conspicuous (Seppälä et al., Reference Seppälä, Karvonen and Valtonen2005). Thus, by injuring important sensory organs, D. spathaceum is able to alter fundamental fish anti-predator mechanisms, such as crypsis and shoaling behaviour, in a way that is likely to increase the vulnerability of the intermediate host to predation by the definitive host (Krause & Ruxton, Reference Krause and Ruxton2002; Seppälä et al., Reference Seppälä, Karvonen and Tellervo Valtonen2004, Reference Seppälä, Karvonen and Valtonen2005, Reference Seppälä, Karvonen and Valtonen2008). Closely related eye flukes, Tylodelphys spp., are also found as metacercariae in the eyes of fish, but these parasites are comparatively understudied for their behavioural impacts (e.g. Vivas Muñoz et al., Reference Vivas Muñoz, Staaks and Knopf2017, Reference Vivas Muñoz, Bierbach and Knopf2019; Ruehle & Poulin, Reference Ruehle and Poulin2019).
The pathological effects of diplostomiasis have seldom been documented in wild fish, partly because heavily infected individuals are removed from the population through predation (Pennycuick, Reference Pennycuick1971). However, D. spathaceum epizootics can be serious in captive fish, particularly farmed salmonids, such as rainbow trout (Oncohynchus mykiss) (Betterton, Reference Betterton1974). Where there is a significant decrease in the Fulton index with increasing levels of parasitism (IRR < 1), then this might indicate a pathogenic effect of the parasite. In the present study, we found that the intensity of infection of carp, Caspian roach and Volga pike perch with Diplostomum spp. was inversely associated with the Fulton index. Other parasites such as A. schupakovi and the Volga pike perch also had an inverse association of infection abundance with the Fulton index. In contrast, the reason for an association between an increasing Fulton index and increasing parasites is not clear.
For a number of parasites, there is a relationship between parasite abundance and age and/or between the proportion that is zero-inflated and age. In some instances, such as the infection of carp with D. spathaceum, the IRR decreases with increasing age. This may be due to age-related resistance or pathogenic effects of the parasite, with heavily infected fish having an excess mortality rate compared to lightly or non-infected fish. The experimental infection of rainbow trout (O. mykiss) suggested an increase in resistance to reinfection following repeated infection (Karvonen et al., Reference Karvonen, Seppälä and Valtonen2004a). Alternatively, there may be a behavioural reason to explain why older fish are less exposed to the parasite. We also found, for some parasitic infections of fish, that IRR increases with age – an example of this is A. schupakovi infection of the marine sander. This could be due to the parasite being long lived with little concomitant resistance to reinfection; thus, the host accumulates parasites over its lifetime. This age-related dynamic of infection is seen in certain parasitic infections of terrestrial vertebrates such as Echinococcus infection of sheep (Torgerson & Heath, Reference Torgerson and Heath2003).
Of interest is the different patterns of the same parasite distribution in different hosts. For D. gobiorum, the abundance decreases with age in bream (IRR < 1), but increases according to age in carp and the Volga pike perch. The reasons for this are somewhat speculative. This may be due to relative resistance or development of an immune response in bream, which is absent in the other species, or variations in infection pressure that could be due to different behaviours of the host species. In different hosts the same parasite also sometimes had different frequency distributions. For example, D. spathaceum was distributed as a zero-inflated negative binomial distribution in bream and as a zero-inflated Poisson distribution in Caspian roach. This may, in this case, be a statistical artefact. The prevalence of this parasite in bream was 21%, whilst in the Caspian roach it was just 4%. When the prevalence of parasites in the sample is low, the probability of having the individual fish with a large intensity of infection is quite low, even if the underlying distribution is negative binomial. Thus, a small sample size might suggest a Poisson distribution even if the population has a negative binomial distribution. Otherwise, it is necessary to consider variations in host resistance of behaviour between different host species to explain these differences.
The reasons behind the variations in parasite abundance in different years is unknown. Fish were sampled at the same time of year, so seasonal differences in transmission appear unlikely. Likewise, potential differences in the dynamics of the Zhaiyk River compared to the open Caspian Sea are unlikely to be confounding factors as the origin of each fish is considered as a covariate in the analysis. There was a higher abundance for K. sinensis in bream and D. spathaceum in asp in the Zhaiyk River compared to the northern Caspian. This may be due to differences in habitat, temperature or salinity, which promote the transmission of the parasites or affect the abundance of intermediate hosts. For ocular trematodes, increases in intensity were found in the roach from late summer to early winter, with another increase in the spring (Burrough, Reference Burrough1978), so seasonal transmission is possible, but such variations could not be analysed in our data.
Where we found differences in abundances of parasites between male and female fish, it was the males that were more heavily infected. This is in agreement with the general pattern of higher infestation in males across a range of host–parasite systems (Poulin, Reference Poulin1996; Klein, Reference Klein2004), and has been linked to relative immune competence between males and females, which may be linked to hormones. However, there are a few results where female fish have been more heavily parasitised (see, e.g., Karvonen & Lindström, Reference Karvonen and Lindström2018).
Whilst the management of parasites in wild populations of fish would not be feasible, there could be opportunities for management of parasitism in aquaculture with a focus on those parasites that appear to be pathogenic or of zoonotic significance.
Financial support
This is part of the project ‘Assessment of the natural foci anisacidosis of and the risks of epizootics in the shelf zones of the north-eastern part of the Caspian Sea’ and was supported by the 217 budget program of the Ministry of Education and Science of the Republic of Kazakhstan, grant/project number АР05131687 .
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.



