Introduction
Prosthogonimosis was an economically important parasitic disease in free-ranging chicken and has lost its significance under conditions of industrial poultry farming.
Rudolphi (Reference Rudolphi1803) described Fasciola ovata, an egg-shaped, flat trematode of 3.3–4.5 × 2.2 mm in size with the acetabulum two times bigger than the oral sucker. The trematodes found in the bursa of Fabricius of a rook (Corvus frugilegus) were given to him by his friend, JCR Meyer. Later, Rudolphi (Reference Rudolphi1809) described a similar trematode, Distoma cuneatum, in a great bustard (Otis tarda). Distoma cuneatum differed by a more pointed, cuneate shape, and eggs were concentrated posterior to ventral sucker. Rudolphi (Reference Rudolphi1819) listed both species and added for the first species the common magpie (Pica pica), the northern shoveler (Spatula clypeata) and the Eurasian coot (Fulica atra) as further hosts.
Wedl (Reference Wedl1858) gave more details on the morphology of a trematode that he found in the bursa of Fabricius of a common snipe (Gallinago gallinago), a common crane (Grus grus) and a Eurasian coot. He wrongly attributed this parasite to Distoma ovatum.
Another trematode with similar morphology was described in the oesophagus of a chicken (von Linstow, Reference von Linstow1873; von Linstow found five specimens in the oesophagus, a rather unusual location – he pointed out that flukes with a related morphology (D. ovatum) were always reported from the bursa of Fabricius). Compared to previously described species, oral and ventral suckers had a similar size, intestinal caeca reached far behind ventral sucker, vitellaria terminated at posterior end of ventral sucker. Since uterine coils were less dense at the posterior end and the worm had a transparent appearance, the name Distomum pellucidum was chosen. Eggs and tegumental spines of D. pellucidum were longer than in previously known species of the extended genus Prosthogonimus created by Lühe (Reference Lühe1899) for trematodes with a genital pore next to the left anterior end of the oral sucker and with parallel situated testes (in the same year, Looss (Reference Looss1899) proposed the genus Prymnoprion, but his paper was published three days later and for this reason Prymnoprion is treated as a junior synonym to Prosthogonimus (Stiles & Wardell, Reference Stiles and Wardell1902)). Not mentioning D. cuneatum, the author recognized D. ovatum and D. pellucidum as valid species.
By re-examining materials of the Berlin collection, Braun (Reference Braun1902) concluded that P. ovatus, P. pellucidus, P. japonicus and P. rarus belong to the genus Prosthogonimus and restored the status of the fifth species, P. cuneatus.
Already in the mid-1950s, the species inventory of the genus consisted of 23 species (Panin, Reference Panin1957) and Skrjabin (Reference Skrjabin and Skrjabin1961) allocated all 37 known Prosthogonimus species to five subgenera.
Here, we present detailed morphological characteristics and phylogenetic analysis of a new Prosthogonimus species found in the bursa of Fabricius of a Peregrine falcon.
Materials and methods
During a routine clinical examination of a female peregrine falcon in January 2020, a fluke infection was detected in the bursa of Fabricius and a total of 23 moving, pink-coloured trematodes were removed and sent for species determination. Intact flukes were washed in phosphate-buffered saline, stained in an aquatic solution of carmine, dehydrated in rising alcohol concentrations and temporary slides embedded in glycerine were used to take measurements. For scanning electron microscopy (SEM), two flukes fixed in 70% ethanol were dehydrated by increasing concentrations of ethanol, critical-point dried using carbon dioxide (Leica EM CPD300; Leica, Wetzlar, Germany) and subsequently mounted on specimen stubs. Dried specimens were sputter-coated with a 10 nm layer of gold–palladium alloy (Polaron Sputter coater SC 7600; Emitech, Montigny-le-Bretonneux, France) before examination with a scanning electron microscope (Supra 40VP; Zeiss, Oberkochen, Germany).
For molecular analyses, total DNA was extracted from three fluke specimens using a DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany) following the manufacturer's recommendation. We amplified the regions of nuclear (internal transcribed spacer 2 (ITS2)) as well as mitochondrial loci (cytochrome c oxidase subunit 1 (cox1) and NADH dehydrogenase subunit 1 (ND1), respectively), using the specific primers listed in Heneberg et al. (Reference Heneberg, Sitko and Bizoz2015). Polymerase chain reaction (PCR) amplification was done in 25 μL reactions consisting of 12.5 μL of Taq PCR Master Mix (Qiagen), 0.4 μM of forward and reverse primers, 2 μL of DNA template and 8.5 μL of RNase-free water. Thermal protocol included initial polymerase activation at 95°C for 5 min followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 57°C (ITS2 and cox1) or 58°C (ND1) for 30 s, extension at 72°C for 1 min and final extension at 72°C for 7 min. After purification, amplicons were subjected to the Sanger sequencing in both directions using ABI 3130 DNA sequencer (Applied Biosystems, Waltham, USA). Sequence alignment and construction of phylogenetic trees were carried out using MEGA 7 software (Kumar et al., Reference Kumar, Stecher and Tamura2016).
Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) analyses were done for sequences generated from the ITS2, cox1 and ND1 regions for preliminary identification. Program selection was optimized for the algorithm searching for somewhat similar sequences (blastn). Reference sequences were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/), and correlated with Heneberg et al. (Reference Heneberg, Sitko and Bizoz2015). Datasets were constructed using MEGA 7 and aligned using the online version of MAFFT (version 7, https://mafft.cbrc.jp/alignment/server/) with the automated selection mode. The best model for maximum likelihood (ML) analyses for each gene region was selected, and the phylogenetic trees constructed using MEGA 7, with 1000 bootstrap replicas. The models used were as follows: ITS2 region – Kimura two-parameter model with gamma-distributed rates (K2 + G); cox1 and ND1 regions – Hasegawa–Kishino–Yano model with gamma-distributed rates with invariant sites (HKY + G + I). Trees were viewed and edited in MEGA 7 software.
One specimen of Prosthogonimus sp. is deposited in the Meguro Parasitological Museum with the registration number MPM Coll. No. 21737.
Results
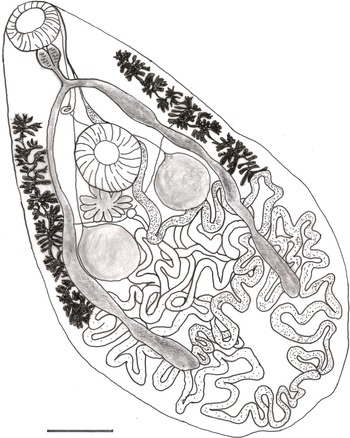
Prosthogonimus falconis n. sp. (figs 1 and 2)
Type specimen. Holotype is deposited in the Meguro Parasitological Museum, Tokyo, Japan, with the registration number MPM Coll. No. 21737

Fig. 1. Prosthogonimus falconis. Scale bar: 0.5 mm.
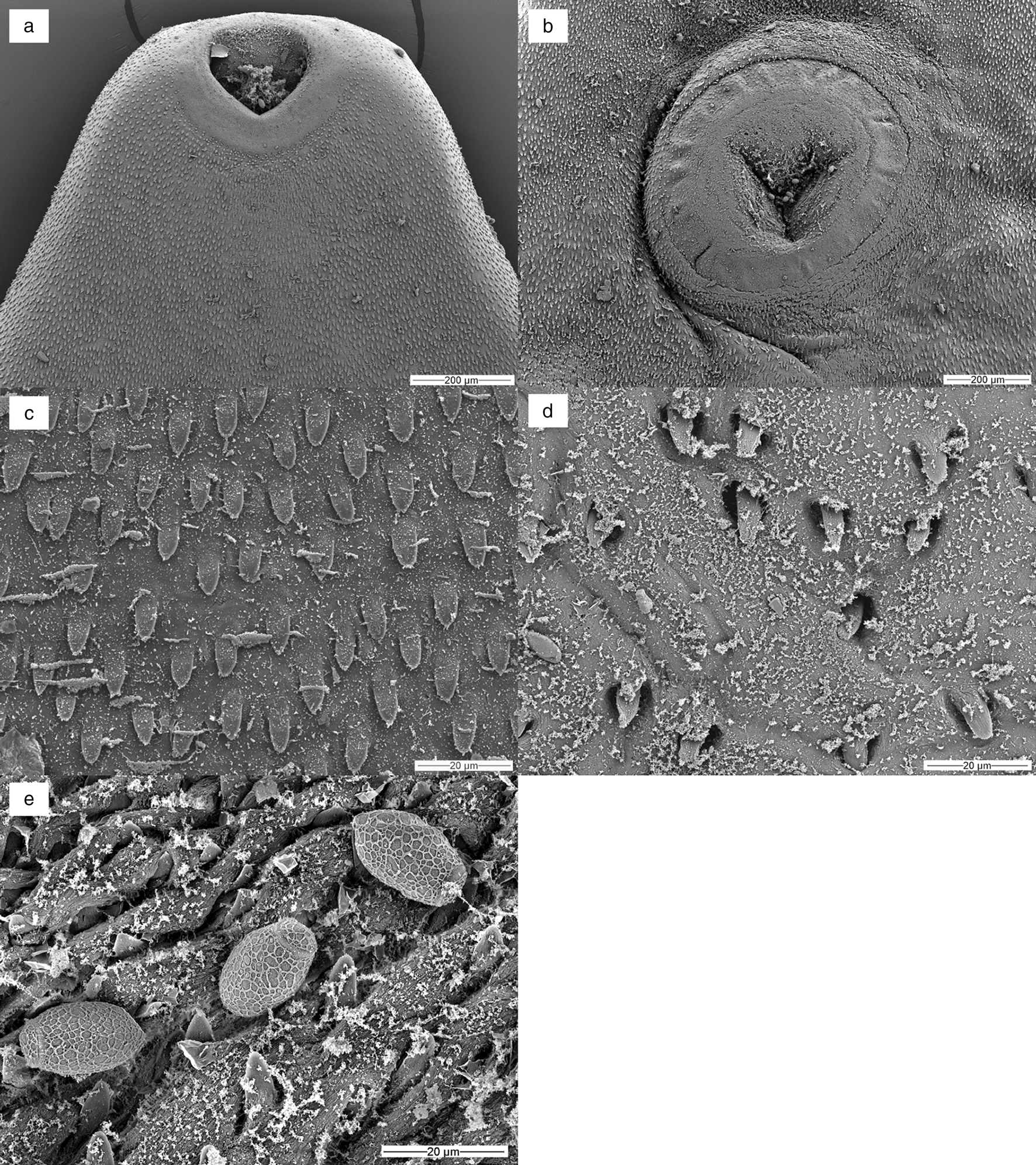
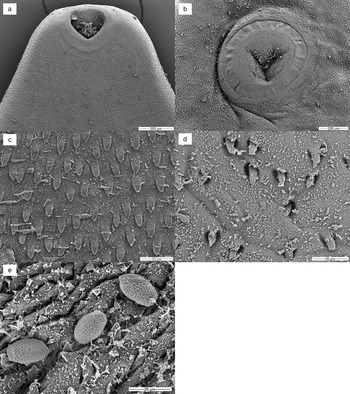
Fig. 2. SEM micrographs of Prosthogonimus sp. from the peregrine falcon: (a) anterior end with oral sucker and cirrus tip – the rim of the oral sucker with rosette-like structures is spineless; (b) ventral sucker; (c) body spines in anterior body part; (d) sparse spination at the posterior end; (e) eggs with net-like structure on the egg shell.
Type host. Peregrine falcon, Falco peregrinus Tunstall, 1771 (Aves: Falconidae).
Site of infection. Bursa of Fabricius.
Intensity of infection. 23.
Type locality. Dubai, Dubai Emirate, United Arab Emirates (UAE) (25°08′28.18″N, 55°20′11.84″E).
Etymology. The species name is derived from the generic name of the host.
Light microscopy
Body flattened, 4.3–6.9 mm long, pear-shaped with greatest width posterior to testes, posterior end rounded (fig. 1 and table 1). Whole body covered with 17–21 μm-long triangle-shaped spines with a base 6–7 μm wide, sparse spination at posterior end. Subterminal oral sucker followed by small pharynx. Acetabulum in anterior half, slightly larger than oral sucker. Oesophagus 155 to 190 μm in length. Intestinal bifurcation in the first fifth of the body. Caeca terminate far posterior to testes in the last fifth of the body. Symmetrical unlobed testes intracaecal. Vasa deferentia unite between anterior rim of ventral sucker and intestinal bifurcation into a short common duct that pass into sausage shaped cirrus sac containing internal seminal vesicle. Common genital pore inconspicuous on left anterior rim of oral sucker. Ovary deeply lobed consisting of 9–11 lobes, posterior or partly dorsal to ventral sucker on opposite body half of genital pore. Seminal receptacle and Mehlis’ gland postovarian. Extracaecal, multifollicular vitelline glands commence prior to acetabulum and terminate posterior to testes. Uterus with descending and ascending coils fill postacetabular space, partly overlapping testes and crossing distal intestinal caeca without forming loops in preacetabular space. Distal uterus as thin tube lateral to cirrus sac. Operculated eggs 21 × 12 μm.
Table 1. Morphology of four Prosthogonimus species according to Heneberg et al. (Reference Heneberg, Sitko and Bizoz2015) and own data.
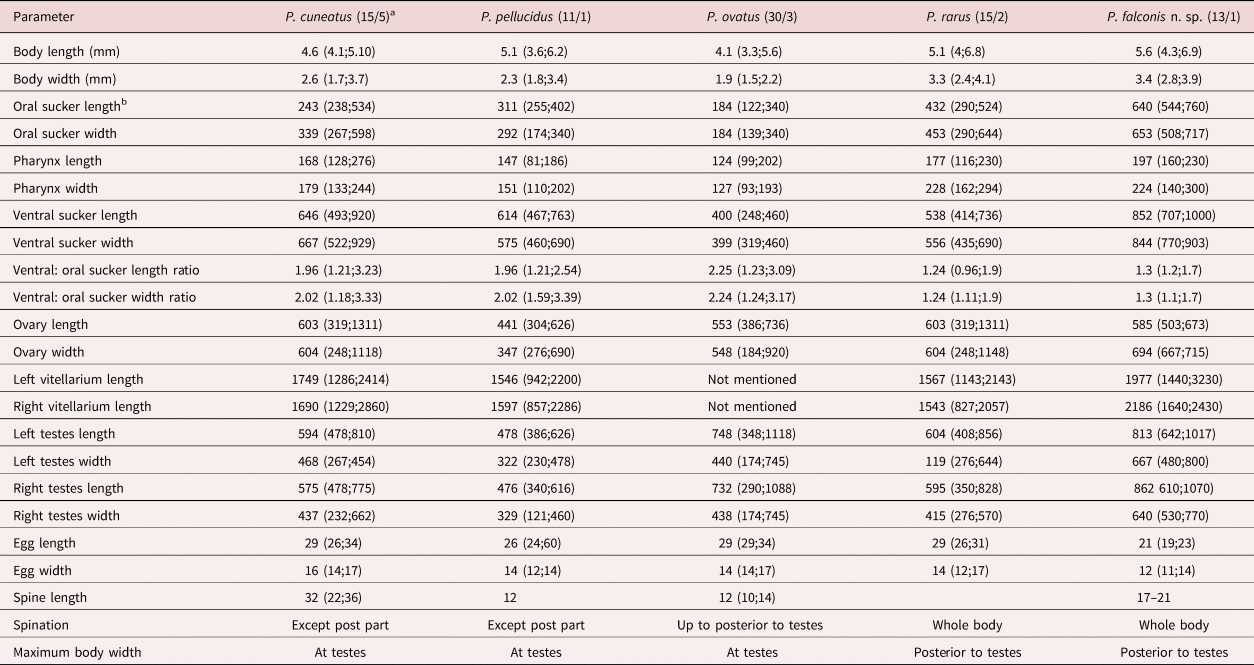
a Numbers in the parentheses represent the number of examined specimens/number of examined hosts
b All measurements are in μm except for body length and body width, which are given in mm
SEM
Rim of the oral sucker with rosette-like structures, spineless. Rim of the ventral sucker with sparse spination. Body spines 10–11 μm long, spines at posterior body less dense. Egg shell covered with the net-like structure of irregular pore size (fig. 2a–e).
Molecular examination
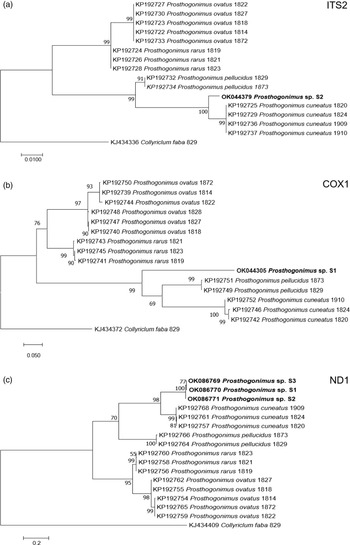
Sequencing of PCR products generated ITS2 (585 bp), cox1 (438 bp) and ND1 (449 bp) sequences, which were deposited to GenBank under accession numbers OK044379, OK044305 and OK086769–OK086771, respectively. Three polymorphic sites with single nucleotide polymorphisms were detected within ND1 sequences of our three analysed fluke specimens. However, all mutations were silent, causing no change in the amino acid sequence.
Nucleotide BLAST analyses showed the highest similarity of our ITS2, cox1 and ND1 sequences to the respective sequences of P. cuneatus (98.5%, 84.2% and 85.4%, respectively) from GenBank (accession numbers KP192725, KP192742, KP192757). In addition, ML analyses of individual sequences placed our fluke sample in a distinct clade, closely related to P. cuneatus and P. pellucidus clusters (fig. 3).
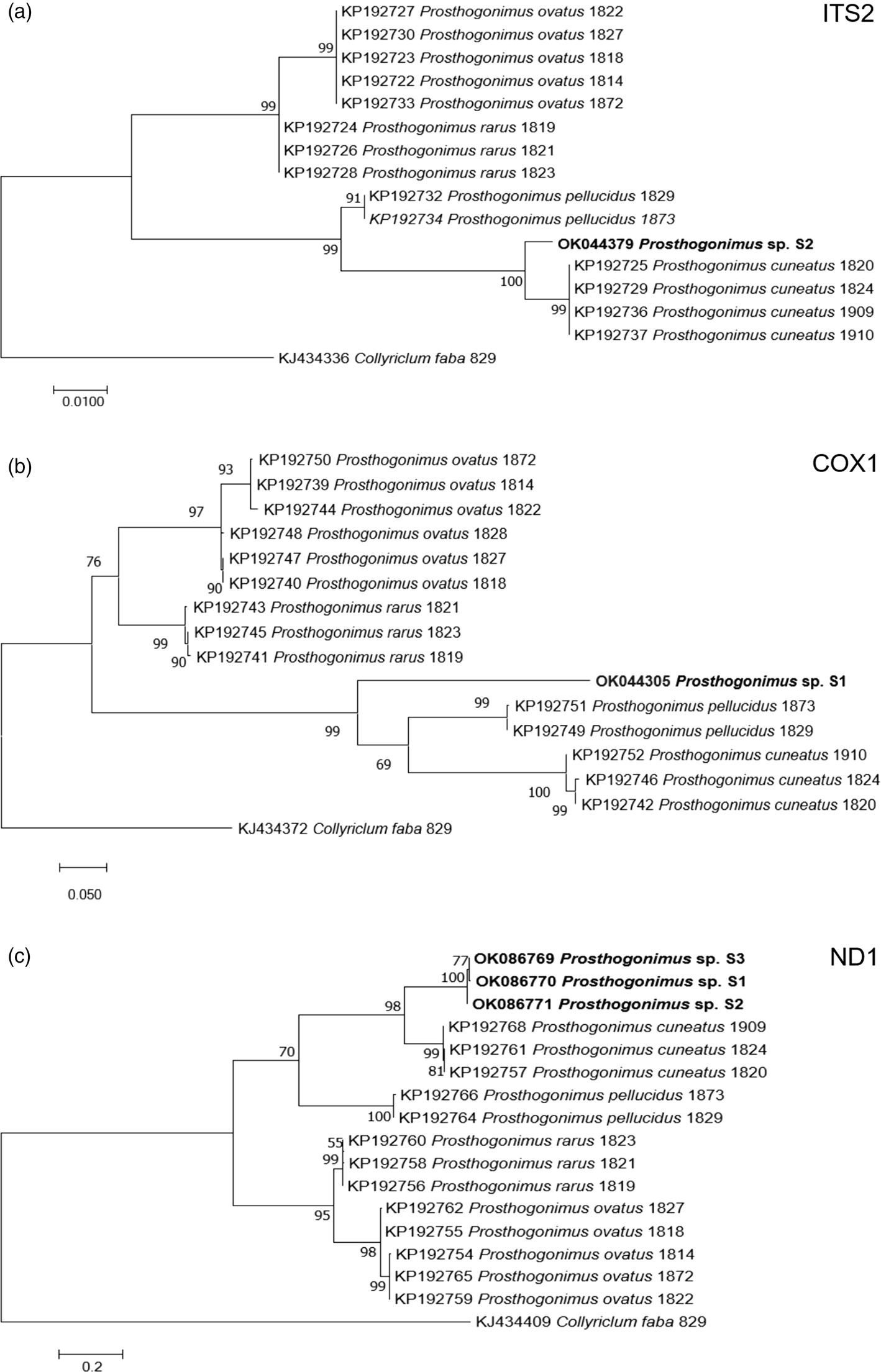
Fig. 3. ML analysis of Prosthogonimus spp. sequences from GenBank. (a) ITS2; (b) cox1; (c) ND1. Sequences generated from the specimens analysed in our study are bolded. The bar represents percentage of genetic variation.
Discussion
Based on the morphology, it is difficult allocating our findings to any of the many described Prosthogonimus species. The paper of Heneberg et al. (Reference Heneberg, Sitko and Bizoz2015) contains not only one of the most comprehensive morphological descriptions but also offers molecular data for four European Prosthogonimus species. In our case, P. ovatus can be excluded as the potential species since uterine coils in P. ovatus fill the entire space between vitelline glands, including the space dorsal to and in front of the ventral sucker; moreover, in P. ovatus body spination discontinues at the posterior edge of testes. Also, the suckers of P. ovatus are relatively small. Oral suckers in P. ovatus, P. cuneatus and P. pellucidus are roughly two times smaller than ventral suckers, while in P. rarus and in our material this relationship was 1:1.24 and 1:1.3, respectively. The maximum body width in P. ovatus, P. cuneatus and P. pellucidus is at the level of the testes, while in P. rarus and our material it is posterior to the testes. Prosthogonimus rarus differs by long intestinal caeca, intracaecal uterine loops and broad vitelline glands that partly overlap the caeca. Our Prosthogonimus specimens from a falcon reveal tegumental spines also on distal body, although spination was less dense and spines were 17–21 μm long by light microscopy, comparable to those of P. rarus (17–24 μm). Measurements on the SEM image revealed shorter lengths, but this is most likely the case because the bases of the spines are deeply embedded in the tegument covering parts of the entire surface. The SEM image of Prosthogonimus eggs showed a net-like structure on the shell similar to other small trematode eggs (Shin et al., Reference Shin, Lim and Choi2009; Lee et al., Reference Lee, Jung, Lim, Lee, Choi, Shin and Chai2012). Eggs in the distal uterus in our material measured only 23 × 14 μm, closest to the eggs from P. cuneatus (26 × 14 μm), and they were considerably smaller than those of other species. With regards to other European Prosthogonimus species, our material can be distinguished from Prosthogonimus longusmorbificans, which has a large body length of 14–16 mm, nearly equal dimension of suckers and short vitellaria, from Prosthogonimus macrorchis, which has testes larger than ventral sucker and very short vitellaria, and from Prosthogonimus limani with a differently structured uterus.
According to Skrjabin's (Reference Skrjabin and Skrjabin1961) division, F. falconis would belong to the subgenus Macrogenotrema Skrjanin & Basakov, Reference Skrjabin and Baskakow1925 in which P. cuneatus was assigned as type species. This subgenus combines species with well-developed reproductive organs and a uterus that forms lopes only posterior to the ventral sucker and these lopes overlay blind-ending caeca. Five species – namely, Prosthogonimus hyperabadensis Jaiswal, 1957, Prosthogonimus indicus Srivastava, 1938, Prosthogonimus ketupi Jaiswal, 1957, Prosthogonimus macroacetabulus Chauhan, 1940 and Prosthogonimus singhi Jaiswal, 1957 – were described from India as a country relatively close to the UAE. From other countries close to the UAE, P. cuneatus was found in Falco tinnunculus (Mohammed, Reference Momammed1999) and recently, Saeed et al. (Reference Saeed, Das Sanjota, Ghazi and Khan2019) reported a new species, Prosthogonimus jonesae found in Vanellus indicus in Pakistan. Sadaf et al. (Reference Sadaf, Javid and Hussain2021) claimed that they found eggs of P. ovatus and P. macrorchis in faecal smears of domestic birds in Punjab, Pakistan in a prevalence of 12.1% and 9.1%, respectively. A curious finding of P. macrorchis in the albumen of an egg was described by Naem & Golpayegani (Reference Naem and Golpayegani2003) in Iran. The other species reported from Iran was P. ovatus, found in P. Pica in a prevalence of 11.3% (Halajian et al., Reference Halajian, Eslami, Mobedi, Amin, Mariaux, Mansoori and Tavakol2011).
All the named species here are morphologically different from P. falconis (table 2).
Table 2. Morphology of Prosthogonimus species described from India, Pakistan and Kazakhstan in comparison to Prosthogonimus falconis (measurements are in mm unless stated otherwise).
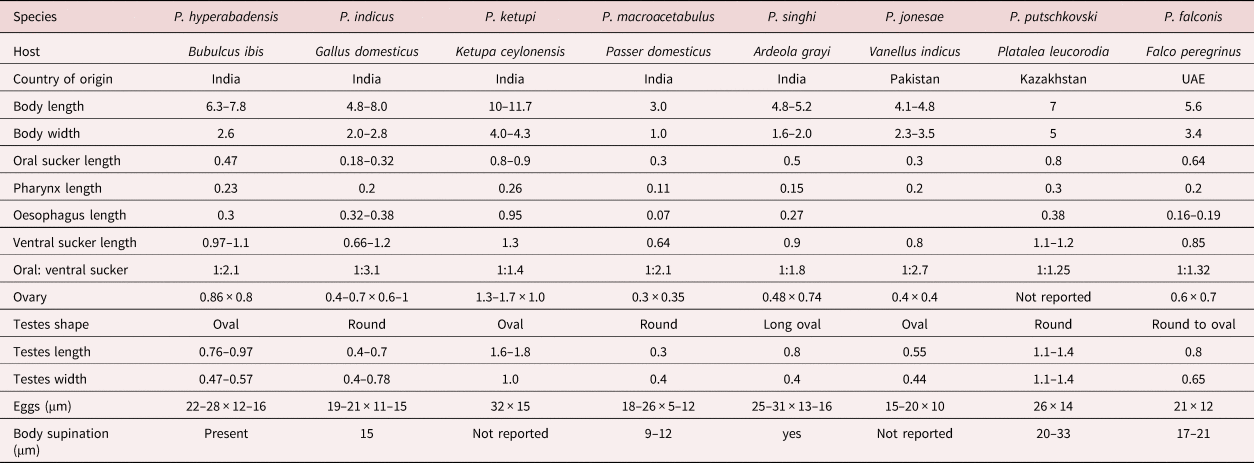
Prosthogonimus hyperabadensis, P. indicus and P. macrorchis differ as they have an elongated body size. The dimensions of P. ketupi are considerably larger, those of P. macrorchis smaller than those of P. falconis. Tegumental spines of P. indicus and P. macorchis are shorter and not reported for P. jonesae. In P. hyperabadensis, P. indicus, P. macrorchis and P. jonesae relation of ventral to oral suckers are >1:2 and eggs of P. ketupi, P. singhi and Prosthogonimus putschkovski are larger than those of F. falconis.
Our newly determined gene sequences showed the highest similarity to those of P. cuneatus. However, percent identity between compared sequences ranged from 84.2% to 98.5%, overcoming intraspecies variation within P. cuneatus and suggesting that our sample belongs to a different species. In addition, phylogenetic dendrograms that were constructed according to ITS2, cox1 and ND1 sequences, designated our sample as a separate species. However, we cannot be sure if it represents a new Prosthogonimus species or one of the existing species that was described only based on morphological criteria, but without molecular confirmation. Therefore, future studies on Prosthogonimus spp. should also include sequencing data in order to corroborate the validity of morphological identification and avoid any bias in species identification.
Since members of the genus Prosthogonimus have a semiaquatic life cycle with prosobranch snails as first and dragon- and damselflies as second intermediate hosts, prosthogonimosis was more often diagnosed in water birds with water as natural habitat (Anseriformes, Ralliformes, Charadriformes, Lariformes), but also in birds of the orders Passerifomes and Galliformes. Only very few infections of birds of prey have been recorded so far from Poland (Falco subbuteo by von Siebold, Reference von Siebold1836; Accipiter nissus and F. subbuteo by Sulgostowska & Czaplinska, Reference Sulgostowska and Czaplinska1987), Russia (F. tinnunculus and Falco vespertinus by Skrjabin & Baskakow, Reference Skrjabin and Baskakow1925), Iraq (F. tinnunculus Moammed, Reference Momammed1999), the Netherlands (F. subbuteo by Borgsteede et al., Reference Borgsteede, Okulewicz, Zoun and Okulewicz2003) and Czech Republic (F. subbuteo by Heneberg et al., Reference Heneberg, Sitko and Bizoz2015).
Unfortunately, neither information on the origin nor on the history of the infected peregrine falcon could be obtained. Birds become infected by ingesting dragonflies or damselflies or their larval stages. According to a survey by Lambret et al. (Reference Lambret, Boudot, Chelmick, De Knijf, Durand, Judas and Stoquert2017), some of the genera of damselflies, hawkers and skimmers that can act as second intermediate hosts are present in the UAE.
As far as it is known, prosobranch snails of the genus Bithynia (Bithynia tentaculta and Bithynia leachi complex) are known as first intermediate hosts. These snails are quite common in the northern Palearctic, and representatives of the genus Bithynia occur in central Asian republics, in Iran and India, but they are absent in the UAE (Feulner & Green, Reference Feulner and Green1999).
Since hunting with falcons is restricted in the UAE, many falconers go on hunting trips to Pakistan, Iran, Afghanistan and central Asian republics or to Morocco where they can find bigger concentrations of bustards, and it is quite possible that the infection was acquired in one of these countries. Maralbayeva & Akhmetov et al. (Reference Maralbayeva and Akhmetov2019) examined birds in Kazakhstan and detected only two members of the genus Prosthogonimus – P. rarus and P. cuneatus – while Khasanova (Reference Khasanova2019) reported only P. ovatus for Uzbekistan.
Acknowledgements
The authors are grateful to Mrs Viertel from the Institute for Zoo and Wildlife Research, Berlin, for her excellent technical assistance for SEM. We thank Dr B. Neuhaus from Berliner Naturkundemuseum for sending copies of difficult-to-obtain historical references.
Financial support
This study was partially supported by the UAE University in Al Ain, UAE (grant numbers 31F095 and G00002877).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.