Introduction
Nematodes of the family Subuluridae are heteroxeneous parasites present in the intestines or caeca of several orders of birds (Anderson, Reference Anderson2000). However, research on the life history of subulurids is limited (Quentin & Poinar, Reference Quentin and Poinar1973). Of the ten genera of Subuluridae found in vertebrates, only two of the genera have been investigated further than the definitive host (Anderson, Reference Anderson2000; Anderson et al., Reference Anderson, Chabaud and Willmott2009). The most studied of these is Subulura brumpti, which is commonly found in the caeca of Galliformes (Anderson, Reference Anderson2000; Pinto et al., Reference Pinto, Menezes and Gomes2004; Peterson, Reference Peterson and Brennan2007). Subulura brumpti utilizes a variety of arthropod intermediate hosts (Alicata, Reference Alicata1939), has a prepatent period of 45 days (Abdou & Selim, Reference Abdou and Selim1963) and has pathological consequences (Nagarajan et al., Reference Nagarajan, Thyagarajan and Raman2012). Although another subulurid, Aulonocephalus pennula, has a higher prevalence and abundance in some Galliformes than S. brumpti, surprisingly little is known about its life history (Peterson, Reference Peterson and Brennan2007).
Aulonocephalus pennula was first described by Chandler (Reference Chandler1935) as an intestinal parasite residing predominantly in the caeca, and a study of subulurid head anatomy suggested that A. pennula was capable of attaching (Inglis, Reference Inglis1958). Although additional studies are needed to determine the pathogenicity of A. pennula, it has been associated with gross pathology (Rollins, Reference Rollins1980), distension of the caeca (Olsen & Fedynich, Reference Olsen and Fedynich2016) and lack of digesta within the caeca (Dunham et al., Reference Dunham, Henry, Brym, Rollins, Helman and Kendall2017a). It has also been hypothesized that A. pennula impairs caecal function, which may lead to malnutrition and negatively affect reproduction, especially during drought years (Rollins, Reference Rollins1980; Lehmann, Reference Lehmann1984; Dunham et al., Reference Dunham, Henry, Brym, Rollins, Helman and Kendall2017a).
This is concerning as the caecum has a variety of functions thought to be important during times of stress, such as absorption of water, breakdown of cellulose, nitrogen absorption and immune response (Fenna & Boag, Reference Fenna and Boag1974; Clench & Mathias, Reference Clench and Mathias1995). Other parasites have been known to increase nitrogen output, reduce fecundity and survival, and cause gross pathology within the caecum (Lehmann, Reference Lehmann1984; Hudson et al., Reference Hudson, Dobson and Newborn1992; Greiner & Ritchie, Reference Greiner, Ritchie, Ritchie, Harrison and Harrison1994; Petkevičius, Reference Petkevičius2007). For example, the helminth Obeliscoides cuniculi reduces survival in the snowshoe hare (Lepus americanus) (Murray et al., Reference Murray, Cary and Keith1997) and Trichostronylus tenuis can regulate red grouse (Lagopus lagopus scoticus) populations by reducing fecundity (Hudson et al., Reference Hudson, Dobson and Newborn1998). Furthermore, the caecal worm, A. pennula, has recently been associated with a die-off of northern bobwhite quail (Colinus virginianus) in Texas (Henry et al., Reference Henry, Brym and Kendall2017).
Bobwhites are economically important game birds that have been experiencing a decline throughout their range (Johnson et al., Reference Johnson, Rollins and Reyna2012; Sauer et al., Reference Sauer, Link, Fallon, Pardieck and Ziolkowski2013). Habitat loss, fragmentation and land-use changes are typically attributed to this decline (Rollins, Reference Rollins and Brennan2007; Hernández et al., Reference Hernández, Brennan, DeMaso, Sands and Wester2013). However, bobwhites are declining at a similar rate in regions considered to have a suitable habitat, such as the Rolling Plains ecoregion of Texas (Rollins, Reference Rollins and Brennan2007; Xiang et al., Reference Xiang, Guo, Zhang, LaCoste, Rollins, Bruno, Fedynich and Zhu2013), suggesting that other factors may be influencing bobwhite populations. In spite of urging by Robel (Reference Robel1993) and Brennan (Reference Brennan2002) for further research regarding factors such as parasitism, there has been a lack of studies on the impacts of parasites (Peterson, Reference Peterson and Brennan2007). When the 2010 quail populations of the Rolling Plains failed to irrupt under favourable conditions, the initiative Operation Idiopathic Decline (OID) investigated the potential role parasites may be playing in the decline. One finding from OID was the abundance of the caecal worm, A. pennula, which infected 73% of bobwhites (Bruno, Reference Bruno2014), and Dunham et al. (Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017b) later reported that infection rate was as high as 99% within some areas of the Rolling Plains.
Although A. pennula has been reported in bobwhites from Texas since 1941 (Webster & Addis, Reference Webster and Addis1945), no studies have determined the intermediate host (Lehmann, Reference Lehmann1953, Reference Lehmann1984; Demarais et al., Reference Demarais, Everett and Pons1987; Dunham et al., Reference Dunham, Henry, Brym, Rollins, Helman and Kendall2017a, b). This is likely due to traditional techniques, such as dissection, being labour intensive and lacking sensitivity (Watts et al., Reference Watts, Courtney and Reddy1999; Kozak & Wędrychowicz, Reference Kozak and Wędrychowicz2010). However, advances in molecular tools can increase sensitivity and are more time efficient and cost effective, particularly in species that lack host specificity (Gasser, Reference Gasser2006; Thompson et al., Reference Thompson, Traub, Parameswaran, Murrell and Fried2007). Because subulurids lack intermediate host specificity (Anderson, Reference Anderson2000), molecular tools such as polymerase chain reaction (PCR) could be valuable when investigating the life cycle of A. pennula. The aim of this study was to identify potential intermediate hosts of A. pennula using PCR.
Materials and methods
Field sampling
Sampling was focused on orthopterans (grasshoppers, crickets and katydids) because A. pennula is thought to have an insect intermediate host (Anderson, Reference Anderson2000; Peterson, Reference Peterson and Brennan2007) and orthopterans are important in the bobwhite diet (Hernández & Peterson, Reference Hernández, Peterson and Brennan2007). Orthopterans were collected in Mitchell County, Texas, as previous research has shown a high prevalence of A. pennula in bobwhites (Dunham et al., Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017b; Henry et al., Reference Henry, Brym and Kendall2017). Orthopterans were collected by sweep nets and by hand on two transects from May 2016 to July 2016. After collection, orthopterans were placed in individual bags and identified within 24 h, using an online key (Brust et al., Reference Brust, Thurman, Reuter, Black, Quartarone and Redford2014) and a field guide (Capinera et al., Reference Capinera, Scott and Walker2004). Following identification, they were stored frozen until DNA extraction could be conducted.
Genomic DNA extractions from insects/intermediate host
Prior to DNA extraction, samples were pooled (based on identification and month of collection) because this has been shown to be an effective method for screening large samples in other species (Goodman et al., Reference Goodman, Orelus, Roberts, Lammie and Streit2003; Guevara et al., Reference Guevara, Vieira, Lilley, Lopez, Vieira, Rumbea, Collins, Katholi and Unnasch2003). The number in each pooled group was based on size and amount collected. Each pooled group was weighed and then homogenized by finely chopping with a scalpel blade or a blender, depending on size. The head, wings and legs of adult grasshoppers were cut off prior to homogenizing. This was done because subulurids are found within the body cavity of the insect (Anderson, Reference Anderson2000), and it would allow for better lysing during the extraction process. DNA was extracted from samples using Qiagen DNeasy blood and tissue kits (Qiagen, Germantown, Maryland, USA), and a negative extraction sample was used every 20th sample. All eluted genomic DNA and extra tissues were stored at −20°C for further use.
Molecular testing for A. pennula larvae
PCR reactions were done in 10 μl volumes with previously described primers, Apen F (10 μm) and Apen R1 (10 μm) (Kalyanasundaram et al., Reference Kalyanasundaram, Blanchard and Kendall2017), and primers ND2_70F (50 μm) and ND2_149R (50 μm) (Kistler et al., Reference Kistler, Parlos, Peper, Dunham and Kendall2016b) for the housekeeping gene. For each reaction 5 μl of 2× Red dye master mix, 0.25 μl of each primer, 1 μl of nuclease-free water, 2 μl of extracted DNA template and 1 μl of housekeeping template were used. The following PCR program was used: 95°C for 3 min; 30 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s; and finally 72°C for 5 min. A 2% agarose gel stained with ethidium bromide (10 g/ml) was used to visualize the PCR products.
Larval identification in intermediate host
Following molecular testing, in August of 2017 live specimens of species that had tested positive were collected to confirm the presence of larvae. If larvae were found, they were sent for sequencing to confirm that they were indeed A. pennula. For cross-confirmation, the same PCR program was run but with newly designed Oxyspirura petrowi ITS1 primers (oxyITS1F 5′- GCAACGCTACTTATTACCACACC-3′ and oxyITS1R 5′-TGGCAGCATACTTGCATCAATGC-3′). Samples that had tested positive for A. pennula, and for which additional tissue was available, were also examined for larvae. The extra tissue was placed on a slide with 0.1× phosphate-buffered saline (PBS). The slide was then placed under the microscope and examined for larvae. All larvae were collected and then extracted as described above. PCR was also run as described above on DNA extracted from larvae.
Results
Thirty-five species from the order Orthoptera were collected, of which nine species showed amplification for A. pennula DNA. Of the nine species of grasshoppers collected from the subfamily Gomphocerinae, five tested positive for A. pennula larvae; while only two of the 11 species collected for Melanoplinae were positive (table 1). For Oedipodinae, two of the three species tested positive. For both Gomphocerinae and Oedipodinae, the number of positive samples increased from May to July, and Gomphocerinae contained the species Opeia obscura, which was positive most frequently (92%). However, neither of the species collected for the subfamily Cyrtacanthacridinae and family Romaleidae were positive for A. pennula. There were also no positives from the order Ensifera (table 2).
Table 1. Percentage of samples from the suborder Caelifera positive for A. pennula larvae (N = number of samples tested).

Table 2. Percentage of samples from the suborder Ensifera positive for A. pennula larvae (N = number of samples tested).
One live specimen of Mermiria bivittata, five of Eritettix simplex, one of Ageneotettix deorum and three of Trachyrhachys kiowa were also collected. Although the T. kiowa and E. simplex samples from 2016 were not positive, they were collected for live samples because we believed this was potentially due to the small sample size, as they are grass-feeding and the other species from those subfamilies were positive. Of these samples, larvae were found in one M. bivittata, E. simplex and T. kiowa (fig. 1). Sequencing results confirmed that all larvae were A. pennula. Also, for cross-confirmation, all the PCR results were negative for O. petrowi ITS1 primers. Additionally, 54 samples that tested positive and had extra tissue were examined (table 3). Larvae were only found in 11 of these samples.
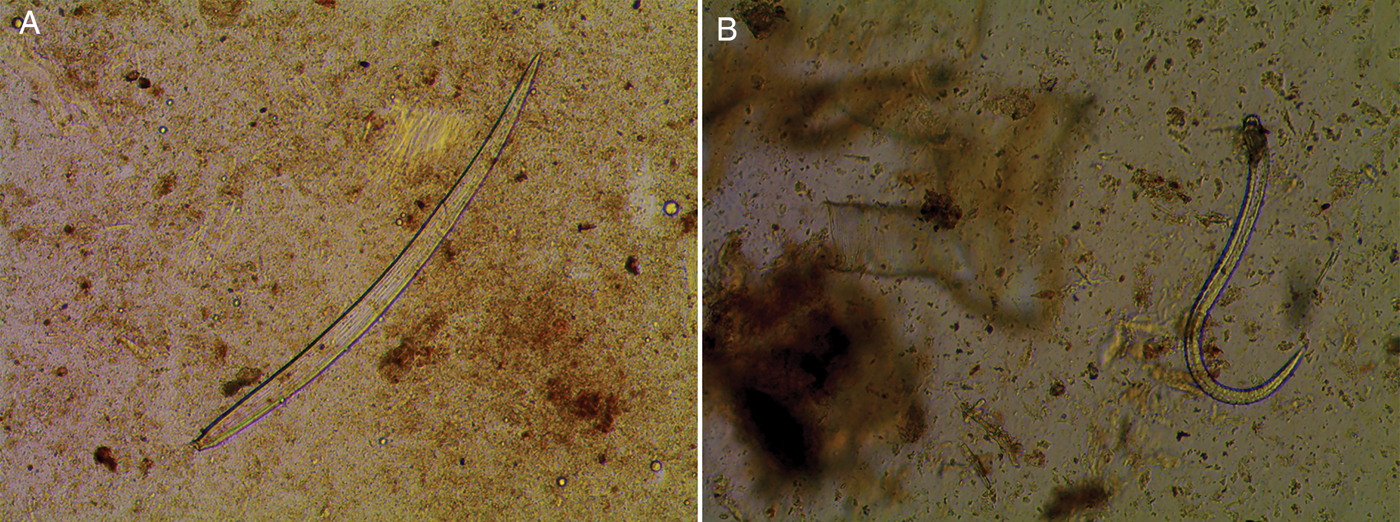
Fig. 1. Third-stage A. pennula larvae from extra tissue of grasshoppers (40×).
Table 3. Percentage of positive samples from samples of the suborder Caelifera with A. pennula larvae present (N = number of samples tested).

Discussion
PCR is often used to confirm that the larvae found during dissections of potential intermediate hosts were indeed from the parasite species of interest or to identify the parasite (Jefferies et al., Reference Jefferies, Morgan and Shaw2009; Lehnert et al., Reference Lehnert, von Samson-Himmelstjerna, Schaudien, Bleidorn, Wohlsein and Siebert2010; Thiengo et al., Reference Thiengo, Maldonado and Mota2010); however, to our knowledge, this is the first study to use PCR as a primary means of identifying potential intermediate hosts, as opposed to dissection followed by PCR. Due to the low specificity of subulurids, which is associated with lower prevalence in an intermediate host (Wetzel & Esch, Reference Wetzel and Esch1996), identifying the intermediate hosts of A. pennula with traditional methods would have proven difficult. Traditional methods are often time consuming and lack sensitivity, as demonstrated here by finding larvae in only 20% of our positive samples. However, molecular techniques have increased sensitivity and make it easier to detect lower infection levels. For example, only 2.9% of Thelazia callipaeda intermediate host were found to be infected following dissection, while 18% were positive with PCR (Otranto et al., Reference Otranto, Lia, Cantacessi, Testini, Troccoli, Shen and Wang2005). In this study, nine potential intermediate hosts from the suborder Caelifera were identified with the use of PCR, and two other potential intermediate hosts were identified during live specimen examination.
Interestingly, all the grasshopper species that tested positive for A. pennula feed primarily on grasses, while all others feed on broadleaf plants or are specialists (Capinera et al., Reference Capinera, Scott and Walker2004). However, not all negative species can be ruled out, due to small sample sizes of some grasshoppers. These results may provide valuable insight into the temporal patterns and spatial distribution associated with parasitic infection that may result from the ecology of these grasshoppers. For instance, prevalence of Echinococcus multilocularis in coyotes was dependent on local variation of the intermediate hosts, and peak infection within the intermediate host determined peak infection within the coyote (Liccioli et al., Reference Liccioli, Kutz, Ruckstuhl and Massolo2014). Similar results were also seen in European eels infected with Partenuisentis ambiguus (Sures & Streit, Reference Sures and Streit2001). This dependence on intermediate host abundance was potentially demonstrated with A. pennula as well. Henry et al. (Reference Henry, Brym and Kendall2017) observed increased infection levels of A. pennula in March 2017 compared to March 2016. It was speculated that this resulted from above-average rainfall and a subsequent increase in arthropod intermediate hosts that facilitated infection in late autumn.
As it has been demonstrated that grass-feeding grasshopper density will likely increase with more precipitation (Lenhart et al., Reference Lenhart, Eubanks and Behmer2015), the potential intermediate hosts identified in this study may increase the prevalence of A. pennula. Furthermore, Guo et al. (Reference Guo, Hao, Sun and Kang2009) showed that temperature increases can expand grasshopper range northward, possibly expanding the distribution of A. pennula. Because precipitation and temperatures are expected to increase in North America as a result of climate change and increase in the frequency of extreme climatic events (Harvell et al., Reference Harvell, Mitchell, Ward, Altizer, Dobson, Ostfeld and Samuel2002; Trenberth, Reference Trenberth2011), there is concern that this will facilitate an increase in the distribution of A. pennula. These climate changes are also expected to result in more epizootic events and prolong their duration and severity in temperate regions (Harvell et al., Reference Harvell, Mitchell, Ward, Altizer, Dobson, Ostfeld and Samuel2002; Hudson et al., Reference Hudson, Cattadori, Boag and Dobson2006). Additionally, Hudson et al. (Reference Hudson, Cattadori, Boag and Dobson2006) speculated that these epizootic events will result from the synchronized emergence of parasites from arrested development, which is associated with unfavourable conditions such as extreme climatic events.
The potential alterations to parasite prevalence and distribution in families related to A. pennula, such as Ascarididae and Anisakidae (Kalyanasundaram et al., Reference Kalyanasundaram, Blanchard and Kendall2017), due to climate change, are already being noted. For example, the ascarid Toxocara canis is becoming more common at higher latitudes (Jenkins et al., Reference Jenkins, Schurer and Gesy2011). The latter authors found that 5% of dogs are now infected with T. canis, while <1% were infected previously. Kim et al. (Reference Kim, Pyo, Hwang, Park, Hwang, Chai and Shin2012) determined that increased temperatures accelerate the development of another ascarid, Ascaris suum, and potentially increases its prevalence. Because A. suum and Ascaris lumbricoides, a parasite that shares a close relationship with A. pennula (Kalyanasundaram et al., Reference Kalyanasundaram, Blanchard and Kendall2017), are closely related (Kim et al., Reference Kim, Pyo, Hwang, Park, Hwang, Chai and Shin2012), it is possible that increased temperatures will also increase the prevalence of A. pennula. Additionally, it is expected that parasites infecting terrestrial animals will expand their range northward due to climate change (Harvell et al., Reference Harvell, Mitchell, Ward, Altizer, Dobson, Ostfeld and Samuel2002). However, the effects of climate change on Subuluridae have not been investigated to our knowledge, and future studies on subulurids and climate change may give insight on the effects climate change will have on A. pennula.
Moreover, it will be important to expand the investigation of the intermediate hosts of A. pennula to other orders, because some parasites with low intermediate host specificity are known to utilize more than 60 species from seven orders (Shostak, Reference Shostak2014). The different intermediate hosts utilized by A. pennula will influence timing and intensity of infection, as well as how it will change with global warming. Because all species that tested positive were primarily grass-feeding, further research should consider insects with similar feeding strategies. Additionally, investigating the potential for Coleoptera and Dermaptera to serve as intermediate hosts may be valuable, because it has already been shown that they are utilized as an intermediate host in another subulurid, S. brumpti (Alicata, Reference Alicata1939). The identification of intermediate hosts is also important for epidemiological studies, understanding timing of larval development and accurately simulating infection in the laboratory (Otranto et al., Reference Otranto, Lia, Buono, Traversa and Giangaspero2004, Reference Otranto, Lia, Cantacessi, Testini, Troccoli, Shen and Wang2005; Kistler et al., Reference Kistler, Hock and Hernout2016a). The species identified in this study may be useful for laboratory studies investigating the life cycle A. pennula, such as larval development.
In this study, it was demonstrated that PCR may be a valuable tool for determining intermediate hosts. This is also the first study to document potential intermediate hosts of A. pennula, but additional studies are needed to determine whether these species are capable of transmitting A. pennula to the bobwhite. This will expand our knowledge of the life cycle of A. pennula, which will be key for determining how A. pennula varies in time and space.
Acknowledgements
We thank the owners and employees of our study ranch for allowing access and providing lodging. Thank you to the Wildlife Toxicology Laboratory personnel for their field and laboratory assistance. We also thank all of the reviewers of this manuscript for their time, comments and consideration.
Financial support
This work was supported by the Rolling Plains Quail Research Foundation (grant number 23A470) and Park Cities Quail (grant number 24A175).
Conflict of interest
None.
Ethical standards
All bobwhites were trapped and handled according to Texas Parks and Wildlife permit SRP-0715-095 and consistent with Texas Tech University Animal Care and Use Committee protocol 16071-08.






