Introduction
The intimate association between hosts and parasites inevitably shapes their evolutionary paths. Parasites evolve to maximize their fitness using the energy and resources of their host; simultaneously hosts evolve to mitigate the adverse effects of infection (Sorensen & Minchella, Reference Sorensen and Minchella2001). Parasites can utilize castration to consume the resources their hosts allocate for reproduction towards their own fitness instead (Lafferty & Kuris, Reference Lafferty and Kuris2009). To diminish the negative fitness impacts of parasitic infections, changes in host life-history traits can evolve (Minchella, Reference Minchella1985). In response to the early stages of parasitic castration and threat to survival, infected hosts can increase their current reproductive investment to make up for lost future reproduction (Minchella & LoVerde, Reference Minchella and Loverde1981). This reproductive investment can be allocated towards an increase in the quantity and/or quality of offspring (Duffield et al., Reference Duffield, Bowers, Sakaluk and Sadd2017). This life-history response is termed ‘fecundity compensation’ (Minchella & LoVerde, Reference Minchella and Loverde1981) or ‘terminal investment’ (Duffield et al., Reference Duffield, Bowers, Sakaluk and Sadd2017).
Fecundity compensation due to parasitism has been documented in a variety of organisms including birds, mammals, insects, molluscs and crustaceans (Duffield et al., Reference Duffield, Bowers, Sakaluk and Sadd2017). For example, the house martin bird, Delichon urbica, can be infected by parasitic protists of Haemoproteus and Plasmodium species, resulting in increased mortality but also increased reproductive investment and success by laying earlier and larger clutches (Marzal et al., Reference Marzal, Bensch, Reviriego, Balbontin and De Lope2008). The fruit fly, Drosophila nigrospiracula, can be infected by an ectoparasitic mite, Macrocheles subbadius, which reduces the life span of the host. However, in an effort to compensate for lost future reproduction opportunities, infected males increase their courtship activity (Polak & Starmer, Reference Polak and Starmer1998). Thus, fecundity compensation is a widespread phenomenon and occurs not only in response to parasitic infection, but also to factors such as age, nutrition-dependent conditions and predation that can negatively impact future reproduction (Duffield et al., Reference Duffield, Bowers, Sakaluk and Sadd2017).
While increased reproductive effort through fecundity compensation has been shown to occur as a life-history response to parasitism, it is unclear whether there is a trade-off between the quantity and quality of host offspring (Duffield et al., Reference Duffield, Bowers, Sakaluk and Sadd2017). That is, would the eggs produced during increased reproductive output from fecundity compensation have the same hatching success and survival to maturity compared to eggs produced by uninfected individuals? In the case of increased reproductive output, neither the fecundity compensation hypothesis nor the terminal investment hypothesis dictates the eventual quality of offspring (Duffield et al., Reference Duffield, Bowers, Sakaluk and Sadd2017). In the parasitoid wasp Lysiphlebus orientalis and host soybean aphid Aphis glycines system, infection results in decreased overall fecundity but larger embryos, possibly accounting for the increased reproductive rate seen in those offspring once mature (Kaiser & Heimpel, Reference Kaiser and Heimpel2016). In deer mice, Peromyscus maniculatus, infected by the trematode Schistosomatium douthitti, there is no change in the number of offspring produced, but offspring from infected individuals are born and weaned at heavier masses. This increase in available resources for the offspring can increase their survival and their reproduction potential once they reach maturity (Schwanz, Reference Schwanz2008).
In this current study, the parasite S. mansoni and its intermediate host, the freshwater snail B. glabrata, were utilized to investigate the consequences of parasitic infection on host reproduction and progeny success. Schistosoma mansoni is the aetiological agent for the human parasitic disease schistosomiasis, which affects over 230 million people worldwide. Freshwater snails of the genus Biomphalaria are infected by S. mansoni miracidia that hatch from eggs in human faeces. Once infected, snails release S. mansoni cercariae into the water where they can infect humans and the cycle repeats (Centers for Disease Control and Prevention, 2018).
Biomphalaria glabrata snails are eventually castrated by S. mansoni; however, soon after exposure, there is an increase in host reproductive output (fecundity compensation; Minchella & LoVerde, Reference Minchella and Loverde1981; Faro et al., Reference Faro, Perazzini, dos Corrêa, Mello-Silva, Pinheiro, Mota, de Souza, de Andrade and Júnior2013). Therefore, this project aims to evince the eventual quality of the offspring produced by infected snails to better understand host–parasite coevolutionary interactions. For many species, there is a trade-off between size and number of offspring (Smith & Fretwell, Reference Smith and Fretwell1974). Furthermore, consistent positive directional selection for larger body size has been widely documented, indicating an association between body size and fitness (Kingsolver et al., Reference Kingsolver, Diamond, Siepielski and Carlson2012). If a reproductive trade-off between offspring quality vs. quantity is present, an increase in host reproductive output could be expected to result in decreased offspring quality. Inversely, if no reproductive trade-off is present, an increase in host reproductive output would have no impact on offspring quality. However, a third alternative is possible, where infected individuals exhibit both increase in reproductive output and improved quality of offspring. For this study, it is predicted that the increase in quantity of eggs produced during the fecundity compensation period will reduce the quality of the individual offspring.
Material and methods
Ninety M-line B. glabrata snails measuring between 8 and 10 mm (approximately ten weeks old; Pimentel, Reference Pimentel1957) were randomly assigned to two groups: exposed and unexposed control. Fifty snails were exposed to Naval Medical Research Institute (NMRI) S. mansoni while 40 snails served as unexposed controls. For S. mansoni exposure, infected mice were euthanized in accordance with Purdue Animal Care and Use Committee Protocol # 1111000225 to isolate Schistosoma eggs. The collected eggs were placed in fresh water for approximately 60 min to allow the miracidia (the infective stage for the snails) to hatch. The resulting miracidia were transferred to six-well plates containing snails. Each snail was exposed individually using five miracidia or sham exposed (unexposed group) for 24 h (Gleichsner et al., Reference Gleichsner, Cleveland and Minchella2016).
Snails were kept in individual jars filled with approximately 390 mL well water and were fed lettuce ad libitum. A small piece of Styrofoam was added to each jar to act as a substrate for egg laying. During the duration of the experiment, five snails of the unexposed control group and three snails of the exposed group died. Data from these snails were excluded after death. Beginning at four weeks post-exposure, infection status was monitored weekly for five weeks by checking for the presence of cercariae after snails had been exposed to fluorescent light for one hour (see supplementary material fig. S1 for full experimental timeline). Snails that released cercariae were designated as infected (37 total). Sham exposed snails were checked to ensure they were not infected; these snails were designated as uninfected (40 total).
The reproductive output was compared between infected and uninfected snail parents by measuring the number of offspring as well as the hatching success and successful survival to maturity of their offspring. Each week post-exposure for a total of five weeks (the length of time between exposure and parasitic castration of the infected parent snail), the number of egg masses and the number of eggs in each egg mass laid by each parent was counted to determine reproductive output. This was achieved by carefully removing the egg masses from the Styrofoam and placing them on glass slides. Using a light microscope, the number of eggs were counted. Furthermore, the number of eggs with no or multiple yolks were counted to compare accuracy in laying eggs. Further analysis and results for egg laying accuracy is discussed in the supplementary material available on the journal's website.
In addition, each week (for a total of five weeks or until parasitic castration of the infected parent snail), approximately 30 eggs with yolk were collected from each parent and used to assess hatching success and survival to maturity. Hatching success was determined by placing approximately ten eggs in a well plate and counting the number of snails that hatched within two weeks of being laid. Survival to maturity was calculated as the proportion of snails that grew to 9 mm in diameter out of the initial number of eggs (Théron et al., Reference Théron, Rognon and Pagès1998). This was done by placing approximately 20 eggs in individual jars filled with approximately 120 ml of well water and allowing them to hatch and mature. Snail offspring were fed lettuce ad libitum. Each week, snails were measured using a calliper and any snails 9 mm or larger were recorded and subsequently removed from the jars. Data for snail growth were collected for five weeks before the experiment was terminated (see supplementary material fig. S1 for a complete experimental timeline). All statistics were done using R version 3.6.3 (R Core Team, 2020) and all pairwise comparisons were done using the multivariate t-distribution P-value correction in the emmeans package (Lenth, Reference Lenth2021). For analysis of fecundity compensation, we performed a zero-inflated mixed-effects model with negative binomial error distribution in the glmmTMB package (Brooks et al., Reference Brooks, Kristensen, van Benthem, Magnusson, Berg, Nielsen, Skaug, Maechler and Bolker2017). This was due to the abundance of zeros in our data and data overdispersion. Weekly egg laying data from infected and uninfected snails was compared over the first three weeks after S. mansoni exposure with individual parent snail as a random factor. By week 4, egg laying in infected snails was nearly absent due to castration. Including weeks 4 and 5 in our statistical analysis would obscure the transient increase in reproduction seen with fecundity compensation. As such, only the first three weeks of reproductive data collection was used for analysis (Minchella & LoVerde, Reference Minchella and Loverde1981).
To determine the impact of infection status compared to parent age, we ran our models two ways using the lme4 package (Bates et al., Reference Bates, Maechler, Bolker and Walker2015). First, hatching success and survival to maturity of offspring was analysed using a generalized linear mixed model with binomial error distribution with infection status as a fixed factor and parent snail, maturation jar and week laid (parent age) included as random factors. Second, hatching and survival to maturity were analysed with the week the parents laid the eggs, indicating parent age, as a fixed effect with parent snail, maturation jar and infection status as random factors.
Results
Fecundity compensation
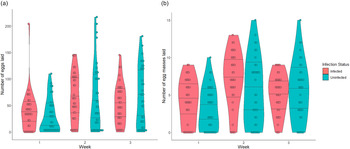
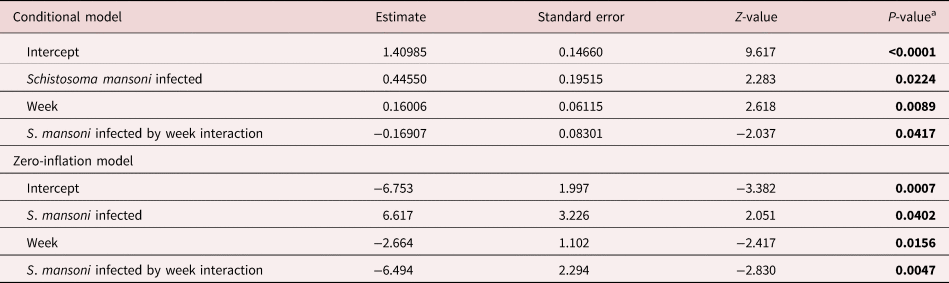
Fecundity compensation in S. mansoni infected B. glabrata snails was dynamic and occurred in two forms. First, the coefficients of our zero-inflation model suggest that infected and uninfected snails have similar probabilities of laying eggs (producing a non-zero egg count) in week 1 post-infection (table 1). However, the significant interaction between infection and week in this model further suggests that in weeks 2 and 3 infected snails have an increased probability of egg laying (table 1 and fig. 1a). The coefficients of the conditional model suggest a similar but flipped relationship with number of eggs laid. Infected snails laid more eggs, especially in weeks 1 and 2, but produced fewer total eggs by week 3 post infection (table 1 and fig. 1a). Similar results are seen when comparing the number of egg masses laid by infected and uninfected snails (table 2 and fig. 1b).

Fig. 1. Violin plot with stacked data points showing the number of eggs laid (a) and number of egg masses laid (b) by Biomphalaria glabrata snails either uninfected (N = 40 snails with 118 total observations) or infected (N = 37 snails with 111 total observations) with Schistosoma mansoni each week after exposure, until week 3 when castration begins for infected snails. Lines within the violin plots delineate the 25%, 50% (median) and 75% data quantiles.
Table 1. Mixed-effects model results with negative binomial error distribution for eggs laid.

a Infected snails are compared relative to uninfected (intercept) with parent snail as a random factor. The estimate of the zero-inflation model represents the probability of obtaining a zero value, or no laying.
Table 2. Mixed-effects model results with negative binomial error distribution for egg masses laid.
a Infected snails are compared relative to uninfected (intercept) with parent snail as a random factor. The estimate of the zero-inflation model represents the probability of obtaining a zero value, or no laying.
Hatching success
We saw that infection status was not a significant predictor of B. glabrata offspring hatching success (proportion hatched from infected parents = 0.816, proportion hatched from uninfected parents = 0.879, P = 0.232, number of observations = 917 offspring from infected + 1323 offspring from uninfected parents). However, the week the parents laid the eggs, indicating parent age and timepoint within the reproductive bout, was a significant predictor of hatching success (P ≤ 0.001; table 3). Eggs that were laid later in the reproductive bout, or older parents, had a greater proportion of offspring hatch (fig. 2).

Fig. 2. Bar graph of week post experiment start, indicating parent snail age (point in reproductive bout), and the proportion of Biomphalaria glabrata offspring hatched out of approximately ten eggs within two weeks of being laid. Number of observations = 433, 592, 591, 325 and 299 in weeks 1, 2, 3, 4 and 5, respectively. P ≤ 0.001.
Table 3. Generalized linear mixed model results comparing weeks post-experiment start (parent snail age) and likelihood offspring hatch with parent snail, maturation jar and infection status as random factors.

The significance of week as a predictor of hatching success suggests offspring originating from later in a reproductive bout (older parents) are more likely to successfully hatch.
Survival to maturity
Infection status played no significant role in the chances of B. glabrata offspring surviving to maturity over the course of the five-week experiment (proportion matured from infected parents = 0.017, proportion matured from uninfected parents = 0.016, P = 0.727). There was also no effect of the week eggs were laid (parent snail age) on survival to maturity (coefficient ± standard error = −0.1976 ± 0.2688, P = 0.459).
Discussion
The interactions between host and parasites result in reciprocal selective pressures, leading to a coevolutionary arms race (Minchella, Reference Minchella1985). Fecundity compensation is a host life-history response to parasitic castration (Minchella & LoVerde, Reference Minchella and Loverde1981). While fecundity compensation is widely documented, little is known about the potential trade-off between the increased number of offspring and the quality of each offspring. In this study, several reproductive measures including reproductive output, hatching success of offspring and successful growth of offspring to maturity were collected. These measures were used to compare offspring produced during infection-induced fecundity compensation to offspring from uninfected snails.
To begin, we ensured that fecundity compensation occurred in our snail host, B. glabrata, as a response to infection with the parasite, S. mansoni. Evidence of fecundity compensation in infected B. glabrata was found in two forms. Earlier, in week 1, infected and uninfected snails had no significant difference in the probability of laying eggs. However, when infected snails did lay, they laid more eggs and egg masses than the uninfected snails. By week 3, infected snails have a higher probability of laying eggs, but the number of eggs and egg masses laid was fewer than uninfected snails. To the best of our knowledge, measures of fecundity compensation have only referred to an increased number or size of offspring produced. This study demonstrated that fecundity compensation can also be viewed as the increased likelihood to produce offspring at all.
The difference in the forms of fecundity compensation may relate to different stages of castration as parasitic castration can be a gradual process (Lafferty & Kuris, Reference Lafferty and Kuris2009). In this study, when infected snails are early in the castration process, they produce more eggs and egg masses when they did lay. Later in the castration process, infected snails lay fewer number of eggs and egg masses, but their likelihood of laying increases. This adaptation may allow the snail host to utilize its reproductive system efficiently, even under challenging circumstances, to produce as many offspring as possible for the next generation.
We predicted a trade-off between the quality and quantity of offspring from infected snails during fecundity compensation vs. uninfected snails during the same time period. However, our results demonstrate that infected snail offspring have the same ability to hatch and survive to maturity as uninfected snail offspring. According to life history theory, resources are limiting and must be budgeted appropriately to remain evolutionarily advantageous (Stearns, Reference Stearns1992). Resources can be allocated towards the short-term growth of an individual, reproduction or long-term health and survival (Sorensen & Minchella, Reference Sorensen and Minchella2001). The lack of a trade-off between offspring quality and quantity in infected vs. uninfected snails implies that exposed snails can allocate enough energy and resources to reproduction and lay healthy eggs that produce viable offspring. However, without an increase in energy/resource intake, increased investment in reproduction must diminish investment to another life-history trait (Sorensen & Minchella, Reference Sorensen and Minchella2001). The increased resources required for fecundity compensation may play a role in the decrease of snail longevity seen during S. mansoni infection (Sturrock & Sturrock, Reference Sturrock and Sturrock1970). In our study, snails were fed ad libitum. Thus, future studies should examine if these results are consistent in resource limited environments.
While infected and uninfected snails showed no difference in the ability of their eggs to hatch, we did see that the later eggs were laid in a reproductive bout, indicating older parents, the more likely they were to hatch. This effect of parental age on reproductive outcomes was only evident when evaluating hatching ability and did not extend to survival to maturity within the duration of our experiment. Similar associations between parental age/size and reproductive performance have been seen in multiple studies with a variety of iteroparous animals (Fox & Czesak, Reference Fox and Czesak2000; Sakai & Harada, Reference Sakai, Harada and McPeek2001; Yanagi & Miyatake, Reference Yanagi and Miyatake2002; Marshall et al., Reference Marshall, Allen, Crean, RN, RJA and JDM2008)). In general, older parents lead to offspring of greater fitness which can be measured in a variety of ways (Marshall et al., Reference Marshall, Heppell, Munch and Warner2010). This includes hatching success as seen with Japanese quail (Coturnix japonica), western gull (Larus occidentalis) and ring-billed gull (Larus delawarensis) (Haymes & Blokpoel, Reference Haymes and Blokpoel1980; Sydeman et al., Reference Sydeman, Penniman, Penniman, Pyle and Ainley1991; Seker et al., Reference Seker, Kul and Bayraktar2004). In snails, this pattern is potentially explained evolutionarily by differences in energy or resource allocation between different ages and stages of development (Gérard & Théron, Reference Gérard and Théron1997). The inclusion of dynamic energy budgets in a reproductive outcome model predicts that younger parents may allocate more resources towards growth and development, but as they become more mature, allocation shifts more towards reproduction (Kooijman, Reference Kooijman1993).
While our results are limited to only a portion of the lifespan of B. glabrata parents, they provide a first step to understanding the overall pattern and optimal parental age for offspring fitness. Future studies should extend the measure of offspring fitness across the entire lifespan of B. glabrata parents and monitor offspring for longer-term success. Offspring fitness can be examined using many other parameters, such as offspring size and life-history traits such as longevity (Plaistow et al., Reference Plaistow, Shirley, Collin, Cornell and Harney2020).
In this study, we explored the consequences of S. mansoni infection on the reproductive success of B. glabrata. In addition to adding further evidence of fecundity compensation, we also expand the definition by showing that it can occur both by increased number of eggs produced as well as increased probability of egg laying. Despite the increase in egg production by exposed hosts, offspring of infected snails appear to do just as well as uninfected snail offspring. Our understanding of host–parasite interactions could benefit from integrating with dynamic energy budget theory to better understand how hosts allocate energy and the optimal strategies for maximizing reproductive output while combating infection (Hall et al., Reference Hall, Becker and Caceres2007; Civitello et al., Reference Civitello, Fatima, Johnson, Nisbet and Rohr2018).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X21000651
Acknowledgements
We would like to sincerely thank the members of the Minchella laboratory group, which without their assistance, completion of this study would not have been possible. Biomphalaria glabrata snails were provided by the NIAID Schistosomiasis Resource Center of the Biomedical Research Institute (Rockville, Maryland) through NIH-NIAID Contract HHSN272201700014I for distribution through BEI Resources.
Financial support
Annabell Davis was supported by a Cable–Silkman Fellowship for undergraduates in Parasitology and the Summer Stay Scholarship provided by Purdue University.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.